Chemistry:Silver sulfite
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
Silver(I) sulfite, Silver sulfite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
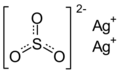
| Ag2O3S | |
| Molar mass | 295.79 g·mol−1 |
| Appearance | White crystals |
| Odor | Odorless |
| Melting point | 100 °C (212 °F; 373 K) decomposes[1][4] |
| 4.6 mg/L (20 °C)[1] | |
Solubility product (Ksp)
|
1.5·10−14[1] |
| Solubility | Soluble in aq. NH4OH, alkali sulfites, AcOH Decomposes in strong acids[2] Insoluble in liquid SO2[3] |
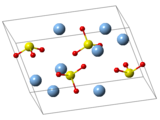
| Structure | |
| Monoclinic, mP24[5] | |
| P21/c, No. 14[5] | |
| 2/m[5] | |
a = 4.6507 Å, b = 7.891 Å, c = 11.173 Å[5] α = 90°, β = 120.7°, γ = 90°
| |
| Hazards | |
| GHS pictograms |  [4] [4]
|
| GHS Signal word | Warning |
| H315, H319, H335[4] | |
| P261, P305+351+338[4] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Silver sulfite is the chemical compound with the formula Ag2SO3. This unstable silver compound when heated and/or in light it decomposes to silver dithionate and silver sulfate.[3]
Preparation
Silver sulfite can be prepared by dissolving silver nitrate with the stoichiometric quantity of sodium sulfite solution, yielding a precipitation of silver sulfite by the following reaction:
- 2 AgNO3 + Na2SO3 ⇌ Ag2SO3 + 2 NaNO3
After precipitation then filtering silver sulfite, washing it using well-boiled water, and drying it in vacuum.[3]
References
- ↑ 1.0 1.1 1.2 Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ Comey, Arthur Messinger; Hahn, Dorothy A. (February 1921). A Dictionary of Chemical Solubilities: Inorganic (2nd ed.). New York City: The MacMillan Company. p. 1046.
- ↑ 3.0 3.1 3.2 Brauer, Georg, ed (1965). Handbook of Preparative Inorganic Chemistry. 2. New York: Academic Press Inc.. p. 1043. ISBN 0323161294. https://books.google.com/books?id=Pef47TK5NfkC&pg=PA1043.
- ↑ 4.0 4.1 4.2 4.3 Sigma-Aldrich Co., Silver carbonate. Retrieved on 2014-07-31.
- ↑ 5.0 5.1 5.2 5.3 Larsson, Lars Olof (1969). "The Crystal Structure of Silver Sulphite". Acta Chemica Scandinavica 23 (7): 2261–2269. doi:10.3891/acta.chem.scand.23-2261.
 |

