Chemistry:Silver thiocyanate
| |
| Names | |
|---|---|
| IUPAC name
Silver(I) thiocyanate, Silver thiocyanate
| |
| Other names
Thiocyanic acid, silver (1+) thiocyanate; Silver isothiocyanate; Silver sulphocyanide[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3077 |
| |
| Properties | |
| AgSCN | |
| Appearance | Colorless crystals |
| Odor | Odorless |
| Melting point | 170 °C (338 °F; 443 K) decomposes[4] |
| 0.14 mg/L (19.96 °C) 0.25 mg/L (21 °C) 6.68 mg/L (100 °C)[1] | |
Solubility product (Ksp)
|
1.03·10−12[2] |
| Solubility | Insoluble in acids (reacts)[3] except when concentrated, acetates, aq. nitrates[1] |
| Solubility in silver nitrate | 43.2 mg/L (25.2 °C, 3 nAgNO3/H2O)[1] |
| Solubility in sulfur dioxide | 14 mg/kg (0 °C)[4] |
| Solubility in methanol | 0.0022 mg/kg[4] |
| −6.18·10−5 cm3/mol[2] | |
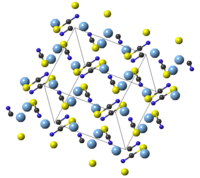
| Structure | |
| Monoclinic, mS32 (293 K)[5] | |
| C2/c, No. 15 (293 K)[5] | |
| 2/m (293 K)[5] | |
a = 8.792(5) Å, b = 7.998(5) Å, c = 8.207(5) Å (293 K)[5] α = 90°, β = 93.75(1)°, γ = 90°
| |
Formula units (Z)
|
8 |
| Thermochemistry | |
Heat capacity (C)
|
63 J/mol·K[4] |
Std molar
entropy (S |
131 J/mol·K[4] |
Std enthalpy of
formation (ΔfH⦵298) |
88 kJ/mol[4] |
| Hazards | |
| GHS pictograms |   [3] [3]
|
| GHS Signal word | Warning |
| H302, H312, H332, H410[3] | |
| P273, P280, P501[3] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Silver thiocyanate is the silver salt of thiocyanic acid with the formula AgSCN. Silver thiocyanate appears as a white crystalline powder. It is very commonly used in the synthesis of silver nanoparticles. Additionally, studies have found silver nanoparticles to be present in saliva present during the entire digestive process of silver nitrate. Silver thiocyanate is slightly soluble in water, with a solubility of 1.68 x 10−4 g/L.[6] It is insoluble in ethanol, acetone, and acid.[7]
Structure
AgSCN is monoclinic with 8 molecules per unit cell. Each SCN− group has an almost linear molecular geometry, with bond angle 179.6(5)°. Weak Ag—Ag interactions of length 0.3249(2) nm to 0.3338(2) nm are present in the structure.[5]
Production
Solution Reaction
Silver thiocyanate has been commonly produced by the reaction between silver nitrate and potassium thiocyanate.[8]
[math]\ce{ AgNO3 + KSCN -> KNO3 + AgSCN }[/math]
Ion-Exchange Route
Silver thiocyanate may be formed via an ion exchange reaction. In this double displacement reaction, silver nitrate and ammonium thiocyanate are dissolved in distilled water to produce silver thiocyanate and ammonium nitrate.[9]
[math]\ce{ AgNO3 + NH4SCN -> NH4 NO3 + AgSCN }[/math]
Additionally, silver thiocyanate can be formed through the double displacement reaction between ammonium thiocyanate and silver chloride to form a precipitate of silver thiocyanate.
[math]\ce{ AgCl + NH4SCN -> NH4 Cl + AgSCN }[/math]
Uses
The most common use of silver thiocyanate is as a silver nanoparticle. Silver thiocyanate nanoparticles have been found in saliva throughout the entire artificial digestion of silver nitrate.[10] The nanoparticles can also be used as good ion conductors.[11]
Silver thiocyanate has also been used to absorb uv-visible light at values less than 500 nm. At longer wavelengths, silver thiocyanate has been found to have good photocatalytic properties.[12]
Characterization[13]
Upon production, silver thiocyanate can be characterized through a wide range of techniques: x-ray powder diffraction (XRD), x-ray photoelectron spectroscopy (XPS), Raman Spectroscopy, ultraviolet photoelectron spectroscopy (UPS), and thermogravimetric analysis (TGA).
Safety[14]
Silver thiocyanate is an irritant to the skin and eyes and is additionally acutely toxic when inhaled dermally or orally. It is sensitive when exposed to the skin as well as toxic to specific organs when singley exposed. Silver thiocyanate is both acutely and chronically hazardous to the aquatic environment.
References
- ↑ 1.0 1.1 1.2 1.3 Comey, Arthur Messinger; Hahn, Dorothy A. (February 1921). A Dictionary of Chemical Solubilities: Inorganic (2nd ed.). New York: The MacMillan Company. p. 884. https://archive.org/details/in.ernet.dli.2015.163725.
- ↑ 2.0 2.1 Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ 3.0 3.1 3.2 3.3 Sigma-Aldrich Co., Silver thiocyanate. Retrieved on 2014-07-19.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Anatolievich, Kiper Ruslan. "silver thiocyanate". http://chemister.ru/Database/properties-en.php?dbid=1&id=8656. Retrieved 2014-07-19.
- ↑ 5.0 5.1 5.2 5.3 5.4 Zhu, H.-L.; Liu, G.-F.; Meng, F.-J. (2003). "Refinement of the crystal structure of silver(I) thiocyanate, AgSCN". Zeitschrift für Kristallographie – New Crystal Structures (München: Oldenbourg Wissenschaftsverlag GmbH) 218 (JG): 263–264. doi:10.1524/ncrs.2003.218.jg.285. ISSN 2197-4578.
- ↑ Kästner, Claudia; Lampen, Alfonso; Thünemann, Andreas F. (2018-02-22). "What happens to the silver ions? – Silver thiocyanate nanoparticle formation in an artificial digestion" (in en). Nanoscale 10 (8): 3650–3653. doi:10.1039/C7NR08851E. ISSN 2040-3372. PMID 29431819.
- ↑ "SILVER THIOCYANATE | 1701-93-5" (in en). https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8348652.htm.
- ↑ "ScienceDirect.com | Science, health and medical journals, full text articles and books.". https://www.sciencedirect.com/?ref=pdf_download&fr=RR-11&rr=82929dfd69f55b22.
- ↑ Zhang, Shuna; Zhang, Shujuan; Song, Limin; Wu, Xiaoqing; Fang, Sheng (2014-05-01). "Synthesis and photocatalytic property of a new silver thiocyanate semiconductor" (in en-US). Chemical Engineering Journal 243: 24–30. doi:10.1016/j.cej.2014.01.015. ISSN 1385-8947. https://www.sciencedirect.com/science/article/abs/pii/S1385894714000321.
- ↑ Kästner, Claudia; Lampen, Alfonso; Thünemann, Andreas F. (2018). "What happens to the silver ions? – Silver thiocyanate nanoparticle formation in an artificial digestion" (in en). Nanoscale 10 (8): 3650–3653. doi:10.1039/C7NR08851E. ISSN 2040-3364. PMID 29431819. http://xlink.rsc.org/?DOI=C7NR08851E.
- ↑ Yang, Ming; Ma, Jing (2009-09-15). "Synthesis and characterizations of AgSCN nanospheres using AgCl as the precursor". Applied Surface Science 255 (23): 9323–9326. doi:10.1016/j.apsusc.2009.07.028. ISSN 0169-4332. Bibcode: 2009ApSS..255.9323Y. https://www.sciencedirect.com/science/article/pii/S0169433209010228.
- ↑ Zhang, Shuna; Zhang, Shujuan; Song, Limin; Wu, Xiaoqing; Fang, Sheng (2014-05-01). "Synthesis and photocatalytic property of a new silver thiocyanate semiconductor" (in en-US). Chemical Engineering Journal 243: 24–30. doi:10.1016/j.cej.2014.01.015. ISSN 1385-8947. https://www.sciencedirect.com/science/article/abs/pii/S1385894714000321.
- ↑ "ScienceDirect.com | Science, health and medical journals, full text articles and books.". https://www.sciencedirect.com/?ref=pdf_download&fr=RR-11&rr=8292abaabc8a81b5.
- ↑ "SILVER THIOCYANATE | 1701-93-5" (in en). https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8348652.htm.
 |


