Chemistry:Strontium selenide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| SrSe | |
| Molar mass | 166.58 |
| Density | 4.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Strontium selenide is an inorganic compound with the chemical formula SrSe.
Preparation
Strontium selenide can be prepared by reducing strontium selenate with hydrogen at 600 °C.[1] It can also be produced by reacting strontium and hydrogen selenide in liquid ammonia.[2]
Properties
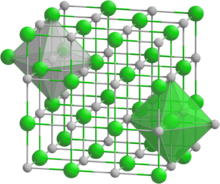
Strontium selenide crystallizes in the orthorhombic crystal system with space group Fm3m. It has a NaCl structure.[3][4] It transforms into a CsCl structure with a space group Pm3m under high pressure (14 GPa).[5]
It reacts with mercury selenide and germanium diselenide at high temperature to obtain the SrHgGeSe4 crystal.[6] It reacts with thorium and selenium at high temperature in the presence of tin to obtain SrTh2Se5.[7]
References
- ↑ A. Henglein (1923-01-26). "Über Erdalkaliselenide" (in en). Zeitschrift für anorganische und allgemeine Chemie 126 (1): 227–236. doi:10.1002/zaac.19231260120. http://doi.wiley.com/10.1002/zaac.19231260120. Retrieved 2021-01-05.
- ↑ T. Petzel, J. Kohle (Dec 1977). "Über die Darstellung von CaSe, SrSe, BaSe und EuSe durch Reaktion der Metalle mit Selenwasserstoff in flüssigem Ammoniak" (in de). Zeitschrift für anorganische und allgemeine Chemie 437 (1): 193–196. doi:10.1002/zaac.19774370127. ISSN 0044-2313. http://doi.wiley.com/10.1002/zaac.19774370127. Retrieved 2021-01-05.
- ↑ Диаграммы состояния двойных металлических систем. 3 Книга 2. М.: Машиностроение. 2000. ISBN 5-217-02932-3.
- ↑ Predel, B. (1998), Madelung, O., ed., "Se-Sr (Selenium-Strontium)" (in en), Pu-Re – Zn-Zr, Landolt-Börnstein - Group IV Physical Chemistry (Berlin/Heidelberg: Springer-Verlag) 5 J: pp. 1, doi:10.1007/10551312_2714, ISBN 978-3-540-61742-6, http://materials.springer.com/lb/docs/sm_lbs_978-3-540-70705-9_2714, retrieved 2023-07-07
- ↑ Purvee Bhardwaj, Sadhna Singh, N.K. Gaur (Mar 2009). "Phase transition, mechanical properties and stability of strontium chalcogenides under high pressure" (in en). Journal of Molecular Structure: THEOCHEM 897 (1–3): 95–99. doi:10.1016/j.theochem.2008.11.033. https://linkinghub.elsevier.com/retrieve/pii/S0166128008007185. Retrieved 2021-01-05.
- ↑ Yangwu Guo, Fei Liang, Wenlong Yin, Zhuang Li, Xiaoyu Luo, Zhe-Shuai Lin, Jiyong Yao, Arthur Mar, Yicheng Wu (2019-04-23). "BaHgGeSe 4 and SrHgGeSe 4 : Two New Hg-Based Infrared Nonlinear Optical Materials" (in en). Chemistry of Materials 31 (8): 3034–3040. doi:10.1021/acs.chemmater.9b01023. ISSN 0897-4756. https://pubs.acs.org/doi/10.1021/acs.chemmater.9b01023. Retrieved 2021-01-05.
- ↑ Amy A. Narducci, James A. Ibers (Jul 1998). "Syntheses, Crystal Structures, and Physical Properties of the New Thorium Chalcogenides CuTh 2 Te 6 and SrTh 2 Se 5" (in en). Inorganic Chemistry 37 (15): 3798–3801. doi:10.1021/ic971594i. ISSN 0020-1669. PMID 11670482. https://pubs.acs.org/doi/10.1021/ic971594i. Retrieved 2021-01-05.
 |
