Chemistry:Sufugolix
 | |
| Clinical data | |
|---|---|
| Other names | TAK-013 |
| Routes of administration | By mouth |
| Drug class | GnRH modulator; GnRH antagonist; Antigonadotropin |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
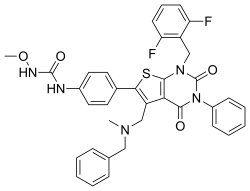
| Formula | C36H31F2N5O4S |
| Molar mass | 667.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sufugolix (INN, BAN) (developmental code name TAK-013) is a non-peptide, orally-active, selective antagonist of the gonadotropin-releasing hormone receptor (GnRHR) (IC50 = 0.1 and 0.06 nM for affinity and in vitro inhibition, respectively).[1] It was under development by Takeda for the treatment of endometriosis and uterine leiomyoma and reached phase II clinical trials for both of these indications, but was subsequently discontinued.[2][3] It seems to have been supplanted by relugolix (TAK-385), which is also under development by Takeda for the treatment of these conditions and has a more favorable drug profile (including reduced cytochrome P450 inhibition and improved in vivo GnRHR antagonistic activity) in comparison.[4]
Oral administration of sufugolix at a dose of 30 mg/kg to castrated male cynomolgus monkeys resulted in nearly complete suppression of luteinizing hormone levels.[1] The duration of action was more than 24 hours, indicating a long elimination half-life of the drug.[1] The suppressive effects of sufugolix on gonadotropin and sex hormone levels are rapidly reversible with discontinuation.[5]
Unlike various other GnRHR antagonists, sufugolix has been elucidated to be a non-competitive or insurmountable/trapping antagonist of the GnRHR rather than a competitive antagonist.[6][7]
See also
References
- ↑ 1.0 1.1 1.2 "Discovery of a thieno[2,3-d]pyrimidine-2,4-dione bearing a p-methoxyureidophenyl moiety at the 6-position: a highly potent and orally bioavailable non-peptide antagonist for the human luteinizing hormone-releasing hormone receptor". Journal of Medicinal Chemistry 46 (1): 113–124. January 2003. doi:10.1021/jm020180i. PMID 12502365.
- ↑ "Selection, synthesis, and structure-activity relationship of tetrahydropyrido[4,3-d]pyrimidine-2,4-diones as human GnRH receptor antagonists". Bioorganic & Medicinal Chemistry 15 (16): 5590–5603. August 2007. doi:10.1016/j.bmc.2007.05.029. PMID 17561404.
- ↑ "Sufugolix - Takeda". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800017215.
- ↑ "Discovery of 1-{4-[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gonadotropin-releasing hormone receptor". Journal of Medicinal Chemistry 54 (14): 4998–5012. July 2011. doi:10.1021/jm200216q. PMID 21657270.
- ↑ "Suppression of a pituitary-ovarian axis by chronic oral administration of a novel nonpeptide gonadotropin-releasing hormone antagonist, TAK-013, in cynomolgus monkeys". The Journal of Clinical Endocrinology and Metabolism 88 (4): 1697–1704. April 2003. doi:10.1210/jc.2002-021065. PMID 12679460.
- ↑ "Trapping of a nonpeptide ligand by the extracellular domains of the gonadotropin-releasing hormone receptor results in insurmountable antagonism". Molecular Pharmacology 72 (2): 238–247. August 2007. doi:10.1124/mol.107.035535. PMID 17409285.
- ↑ "Challenges and opportunities of trapping ligands". Molecular Pharmacology 72 (2): 231–234. August 2007. doi:10.1124/mol.107.038208. PMID 17522183.
External links
 |

