Chemistry:Uranium hexachloride
| |
| Names | |
|---|---|
| IUPAC name
Uranium(VI) chloride
| |
| Other names
Uranium hexachloride
Peruranic chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| UCl 6 | |
| Molar mass | 450.745 g/mol |
| Appearance | dark green crystalline solid |
| Density | 3600 kg/m3 |
| Melting point | 177 °C (351 °F; 450 K) |
| Related compounds | |
Other anions
|
Uranium hexafluoride |
Other cations
|
Tungsten hexachloride |
Related uranium chlorides
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uranium hexachloride (UCl
6) is an inorganic chemical compound of uranium in the +6 oxidation state.[1][2] UCl
6 is a metal halide composed of uranium and chlorine. It is a multi-luminescent dark green crystalline solid with a vapor pressure between 1-3 mmHg at 373.15 K.[3] UCl
6 is stable in a vacuum, dry air, nitrogen and helium at room temperature. It is soluble in carbon tetrachloride (CCl
4). Compared to the other uranium halides, little is known about UCl
6.
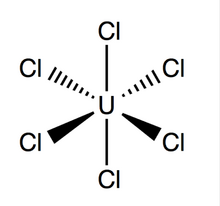
Structure and Bonding
Uranium hexachloride has an octahedral geometry, with point group Oh. Its lattice (dimensions: 10.95 ± 0.02 Å x 6.03 ± 0.01 Å) is hexagonal in shape with three molecules per cell; the average theoretical U-Cl bond is 2.472 Å long (the experimental U-Cl length found by X-ray diffraction is 2.42 Å),[4] and the distance between two adjacent chlorine atoms is 3.65 Å.
Chemical properties
Uranium hexachloride is a highly hygroscopic compound and decomposes readily when exposed to ordinary atmospheric conditions.[5] therefore it should be handled in either a vacuum apparatus or in a dry box.
Thermal decomposition
UCl
6 is stable up to temperatures between 120 °C and 150 °C. The decomposition of UCl
6 results in a solid phase transition from one crystal form of UCl
6 to another more stable form.[6] However, the decomposition of gaseous UCl
6 produces UCl
5. The activation energy for this reaction is about 40 kcal per mole.
- 2 UCl
6 (g) → 2 UCl
5 (s) + Cl
2 (g)
Solubility
UCl
6 is not a very soluble compound. It dissolves in CCl
4 to give a brown solution. It is slightly soluble in isobutyl bromide and in fluorocarbon (C
7F
16).[6]
| Solvents | Temperature (°C) | Grams of UCl 6/100g of solution |
|---|---|---|
| CCl 4 |
−18 | 2.64 |
| CCl 4 |
0 | 4.9 |
| CCl 4 |
20 | 7.8 |
| 6.6% Cl 2 : 93.4% CCl 4 |
−20 | 2.4 |
| 12.5% Cl 2 : 87.5% CCl 4 |
−20 | 2.23 |
| 12.5% Cl 2 : 87.5% CCl 4 |
0 | 3.98 |
| Liquid Cl 2 |
−33 | 2.20 |
| CH 3Cl |
−24 | 1.16 |
| Benzene | 80 | Insoluble |
| Freon 113 | 45 | 1.83 |
Reaction with hydrogen fluoride
When UCl
6 is reacted with purified anhydrous liquid hydrogen fluoride (HF) at room temperature produces UF
5.[6]
- 2 UCl
6 + 10 HF → 2 UF
5 + 10 HCl + Cl
2
Synthesis
Uranium hexachloride can be synthesized from the reaction of uranium trioxide (UO
3) with a mixture of liquid CCl
4 and hot chlorine (Cl
2). The yield can be increased if the reaction carried out in the presence of UCl
5.[7] The UO
3 is converted to UCl
5, which in turn reacts with the excess Cl
2 to form UCl
6. It requires a substantial amount of heat for the reaction to take place; the temperature range is from 65 °C to 170 °C depending on the amount of reactant (ideal temperature 100 °C - 125 °C). The reaction is carried out in a closed gas-tight vessel (for example a glovebox) that can withstand the pressure that builds up.
Step 1: 2 UO
3 + 5 Cl
2 → 2 UCl
5 + 3 O
2
Step 2: 2 UCl
5 + Cl
2 → 2 UCl
6
Overall reaction: 2 UO
3 + 6 Cl
2 → 2 UCl
6 + 3 O
2
This metal hexahalide can also be synthesized by blowing Cl
2 gas over sublimed UCl
4 at 350 °C.[8]
Step 1: 2 UCl
4 + Cl
2 → 2 UCl
5
Step 2: 2 UCl
5 + Cl
2 → 2 UCl
6
Overall Reaction: UCl
4 + Cl
2 → UCl
6
References
- ↑ Zachariasen, W. H. (1948). "Crystal chemical studies of the 5f-series of elements. V. The crystal structure of uranium hexachloride". Acta Crystallographica 1 (6): 285–287. doi:10.1107/S0365110X48000788.
- ↑ Taylor, J. C.; Wilson, P. W. (1974). "Neutron and X-ray powder diffraction studies of the structure of uranium hexachloride". Acta Crystallographica Section B 30 (6): 1481. doi:10.1107/S0567740874005115.
- ↑ Van Dyke, R. E.; Evers, E. C. (1955). "Preparation of Uranium Hexachloride". Google Patents: 2.
- ↑ Batista, E. R.; Martin, R. L.; Hay, P. J. (2004). "Density Functional Investigations of the Properties and Thermodynamics of UFn and UCln (n=1,...,6)". J. Chem. Phys. 121 (22): 11104–11. doi:10.1063/1.1811607. PMID 15634063. https://zenodo.org/record/1231911.
- ↑ Lipkin, D.; Wessman, S. (1955). "Process and Apparatus for protecting Uranium hexachloride from Deterioration and Contamination". Google Patents: 2.
- ↑ 6.0 6.1 6.2 Katz, J.J.; Rabinowitch,E. (1951). The Chemistry of Uranium. Ann Arbor: The McGraw-Hill Book Company.
- ↑ Van Dyke, R. E.; Evers, E. C. (1955). "Preparation of Uranium Hexachloride". Google Patents: 2.
- ↑ Thornton, G.; Edelstein, N.; Rösch, N.; Woodwark, D.R.; Edgell, R.G. (1979). "The Electronic Structure of UCl6: Photoelectron Spectra and Scattered Wave Xα Calculations". J. Chem. Phys. 70 (11): 6. doi:10.1063/1.437313. Bibcode: 1979JChPh..70.5218T.
 |
