Chemistry:Uranium tetrabromide
From HandWiki
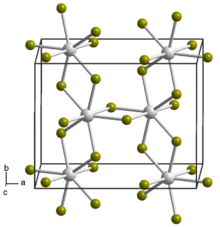
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| UBr4 | |
| Molar mass | 557.645 g/mol |
| Appearance | brown crystalline solid |
| Density | 5190 kg/m3 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H300, H330, H373, H411 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uranium tetrabromide is an inorganic chemical compound of uranium in oxidation state +4.
Production
Uranium tetrabromide can be produced by reacting uranium and bromine:[1]
U+ 2 Br2 → UBr4
References
 |
