Chemistry:Zinc acetylacetonate
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H14O4Zn | |
| Molar mass | 263.60 g·mol−1 |
| Appearance | crystals[1] |
| Density | 1.41 g·cm−3[2] |
| Melting point | 124–126 °C[1] |
| Boiling point | 129–131 °C (13 hPa)[1] |
| 6.9 g/L[1] | |
| Solubility | soluble in organic solvants[3] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P271, P280, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
| Related compounds | |
Other cations
|
calcium acetylacetonate barium acetylacetonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
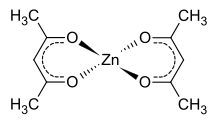
Zinc acetylacetonate is an acetylacetonate complex of zinc, with the chemical formula of Zn(C
5H
7O
2)
2. The compound is in fact a trimer, Zn3(acac)6, in which each Zn ion is coordinated by five oxygen atoms in a distorted trigonal bipyramidal structure.[5]
Preparation
Zinc acetylacetonate can be obtained by reacting zinc sulfate, acetylacetone and sodium hydroxide.[3]
Properties
Zinc acetylacetonate is a crystalline substance that is slightly soluble in water.[1] Through sublimation, monomer crystals can be obtained, which are monoclinic and have the space group C2/c (No. 15).[6] Trimeric crystals can also be obtained by sublimation, which is also monoclinic, with space group C2 (No. 5).[2] The structures of its monohydrate[7] and dihydrate[8] are also known.
Reactions
Zinc acetylacetonate hydrate has been used to prepare magnetic (Zn,Fe)Fe2O4 films,[9] zinc oxide,[10] and is also a catalyst for organic synthesis.[3]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Sigma-Aldrich Co., product no. 8.08803.
- ↑ 2.0 2.1 Bennett, M. J.; Cotton, F. A.; Eiss, R. (1968-07-01). "The crystal and molecular structure of trimeric bis(acetylacetonato)zinc(II)". Acta Crystallographica Section B 24 (7): 904–913. doi:10.1107/S0567740868003390. ISSN 0567-7408. https://scripts.iucr.org/cgi-bin/paper?S0567740868003390.
- ↑ 3.0 3.1 3.2 Barta, Nancy S.; Stille, John R. (2001-04-15), John Wiley & Sons, Ltd, ed. (in en), Bis(acetylacetonato)zinc(II), Chichester, UK: John Wiley & Sons, Ltd, pp. rb097, doi:10.1002/047084289x.rb097, ISBN 978-0-471-93623-7, https://onlinelibrary.wiley.com/doi/10.1002/047084289X.rb097, retrieved 2023-03-06
- ↑ "zinc;(Z)-4-oxopent-2-en-2-olate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/5360437#section=Safety-and-Hazards.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ W. Clegg (2016). Private Communication. Cambridge Crystallographic Data Centre. doi:10.5517/ccdc.csd.cc1mcf4k.
- ↑ H. Montgomery, E. C. Lingafelter (1963). "The crystal structure of monoaquobisacetylacetonatozinc". Acta Crystallographica 16 (8): 748–752. doi:10.1107/S0365110X6300195X.
- ↑ P. Harbach, H.-W. Lerner, M. Bolte (2003). "Diaquadiacetylacetonatozinc(II)". Acta Crystallographica Section E 59 (9): m724–m725. doi:10.1107/S1600536803015848.
- ↑ Sigma-Aldrich Co., product no. 480991.
- ↑ Inubushi, Yoichi; Takami, Ryoji; Iwasaki, Mitsunobu; Tada, Hiroaki; Ito, Seishiro (April 1998). "Mechanism of Formation of Nanocrystalline ZnO Particles through the Reaction of [Zn(acac)2 with NaOH in EtOH"] (in en). Journal of Colloid and Interface Science 200 (2): 220–227. doi:10.1006/jcis.1997.5354. Bibcode: 1998JCIS..200..220I. https://linkinghub.elsevier.com/retrieve/pii/S0021979797953546.
 |

