Medicine:McCune–Albright syndrome
McCune–Albright syndrome is a complex genetic disorder affecting the bone, skin and endocrine systems. It is a mosaic disease arising from somatic activating mutations in GNAS, which encodes the alpha-subunit of the Gs heterotrimeric G protein.[1]
It was first described in 1937 by American pediatrician Donovan James McCune and American endocrinologist Fuller Albright.[2][3][4]
Signs and symptoms
McCune–Albright syndrome is suspected when two or more of the following features are present:
- Fibrous dysplasia (specifically, polyostotic fibrous dysplasia)
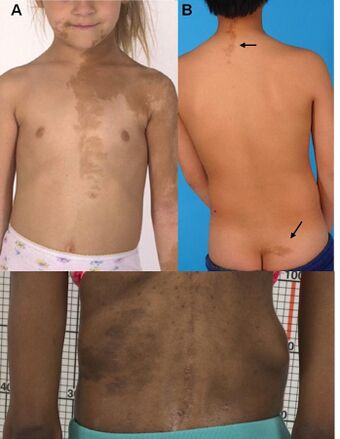
- Hyperpigmented skin lesions with characteristic features, including jagged "coast of Maine" borders and tendency occur along the midline of the body. These lesions are historically termed café au lait macules, however the term "cafe-au-lait" only describes their appearance on lighter-skinned individuals.[5]
- Hyperfunctioning endocrine disease
Patients may have one or many of these features, which may occur in any combination. As such, the clinical presentation of patients with McCune Albright syndrome varies greatly depending on the disease features.[6]
Various endocrine diseases may present in McCune–Albright syndrome due to increased hormone production.
- Precocious puberty: The most common endocrinopathy is precocious puberty, which presents in girls (~85%) with recurrent estrogen-producing cysts leading to episodic breast development, growth acceleration, and vaginal bleeding.[7][8] Precocious puberty may also occur in boys with McCune–Albright syndrome, but is much less common (~10–15%).[8] In children of both sexes, growth acceleration may lead to tall stature in childhood, however premature bone maturation may lead to early growth plate fusion and short stature in adulthood.
- Testicular abnormalities: Testicular abnormalities are seen in a majority (~85%) of boys with McCune–Albright syndrome.[8][9][10] These typically present with macro-orchidism. On pathology lesions show Leydig and Sertoli cell hyperplasia.
- Hyperthyroidism: Hyperthyroidism occurs in approximately one-third of patients with McCune Albright syndrome. Patients have characteristic abnormalities on thyroid ultrasound, and may have a slight increased risk for thyroid cancer.[8][9][10]
- Growth hormone excess: Excess growth hormone secretion and is found in approximately 10–15% of patients.[8] This may lead to expansion of craniofacial fibrous dysplasia, increasing the risk of vision and hearing loss.[11][12]
- Cushing's syndrome: In McCune–Albright syndrome, Cushing's syndrome is a very rare feature that develops only in infancy.[13]
- Hypophosphatemia due to increased fibroblast growth factor 23 production may lead to rickets, osteomalacia, and worsening skeletal outcomes.[6]
Genetics
Genetically, there is a spontaneous postzygotic mutation of the gene GNAS, on the long (q) arm of chromosome 20 at position 13.3, which is involved in G-protein signaling.[14] This mutation, which occurs only in the mosaic state, leads to constitutive receptor signaling and inappropriate production of excess cAMP.[15]
The mutation that causes McCune–Albright syndrome arises very early during embryogenesis. Because all cases of the syndrome are sporadic, it is believed that the mutation would be lethal if it affected all cells in the embryo. Mutant cells can only survive when they are intermixed with normal cells.[16]
There are no known risk factors for acquiring McCune–Albright syndrome, and no exposures during pregnancy that are known to either cause or prevent the mutation from occurring. The disease cannot be inherited and occurs equally among all ethnic groups.[17]
Diagnosis
McCune–Albright syndrome has different levels of severity. For example, one child with McCune–Albright syndrome may be entirely healthy, with no outward evidence of bone or endocrine problems, enter puberty at close to the normal age, and have no unusual skin pigmentation. Diagnosis may be made only after decades. In other cases, children are diagnosed in early infancy, show obvious bone disease, and obvious increased endocrine secretions from several glands.[citation needed]
Skeletal abnormalities
All patients with known or suspected McCune–Albright syndrome should undergo a screening evaluation for fibrous dysplasia.[18] Nuclear medicine tests such as technetium-99 scintigraphy are the most sensitive way to detect fibrous dysplasia lesions.[19]
CT scan of the skull is the most useful test to evaluate craniofacial fibrous dysplasia. Regular hearing and vision screening is recommended. X-rays are usually sufficient to reveal fibrous dysplasia of the appendicular skeleton, but CT and/or MRI scans can reveal microfractures. Regular screening for scoliosis should also be undertaken.[citation needed]
Endocrine abnormalities
Patients with known or suspected McCune–Albright syndrome should undergo a screening evaluation for endocrine features.
Precocious puberty is typically diagnosed based on clinical presentation. A bone age examination should be performed to evaluation for skeletal maturation. Boys and men should have a screening testicular ultrasound.[18]
Hyperthyroidism is diagnosed based on blood tests. A screening thyroid ultrasound exam may be performed.[18]
Growth hormone excess is diagnosed using blood tests, such as insulin-like growth factor-1 levels. Monitoring growth rates alone is not sufficient to screen for growth hormone excess, because linear growth in children with McCune–Albright syndrome may be affected by skeletal deformities and other endocrinopathies.[18]
Hypophosphatemia is diagnosed by blood phosphorus levels.[18]
Cushing syndrome is very rare, and is typically diagnosed clinically in infants who present with signs of severe illness.[18]
Treatment
Treatment is dictated by both the tissues affected and the extent to which they are affected.[17]
Skeletal abnormalities
Surgical management of skeletal abnormalities has evolved over the years. Surgical intervention may be necessary for some skeletal abnormalities. Bisphosphonates are helpful in relieving bone pain, but it is no longer believed that they prevent progression of the disease.[20] Denosumab has been found successful in reducing bone pain and decreasing tumor growth, however there is limited safety data available in patients with fibrous dysplasia.[21] Muscle strengthening exercises are important for preventing bone fractures; cycling and swimming are recommended in order to reduce the risk of fracture during exercise.[17]
Screening and management of endocrinopathies is an important part of managing fibrous dysplasia. For example, untreated growth hormone excess increases the risk of craniofacial fibrous dysplasia expansion and may lead to vision loss.[22] Untreated hyperthyroidism and hypophosphatemia increases the risk of fractures and skeletal deformities.[6]
Endocrine abnormalities
For treatment of precocious puberty, the aromatase inhibitor such as letrozole is effective at prevent bleeding episodes and preventing short stature.[7] In boys, these should be combined with drugs such as spironolactone and flutamide to treat androgen excess.[23] Periodic ultrasounds of testicular lesions should be performed to screen for cancer.[23]
Hyperthyroidism is managed with medications such as thioamides.[24] However, because hyperthyroidism in McCune–Albright syndrome does not resolve, surgery or radiation are more definitive treatments. Periodic thyroid examination should be performed to screen for thyroid cancer.[18]
Oral phosphate and calcitriol may be given for treatment of hypophosphatemia.[18]
For growth hormone excess, treatment with somatostatin analogues or pegvisomant may be effective.[22] Surgery may be an option, but may be complicated by the cranial abnormalities associated with the disorder. Excessive prolactin secretion may also occur; this is treated with dopamine agonists such as cabergoline. Radiation therapy has been associated with malignant transformation of skull base fibrous dysplasia, and should be avoided in all but the most dire cases.[6]
Cushing syndrome is a rare but potentially fatal complication that can occur in the first year of life. Adrenalectomy is the treatment of choice. Metyrapone may also be used for treatment.[17]
Epidemiology
McCune–Albright syndrome is estimated to occur at a frequency between 1 person in 100,000 to 1 person in 1,000,000 individuals worldwide.[25]
See also
- List of cutaneous conditions
- List of radiographic findings associated with cutaneous conditions
References
- ↑ Boyce, Alison M.; Collins, Michael T. (1993). "Fibrous Dysplasia/McCune-Albright Syndrome". in Adam, Margaret P.. GeneReviews. Seattle (WA): University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK274564/.
- ↑ synd/1844 at Who Named It?
- ↑ McCune, Donovan J.; Bruch, Hilde (1937). "Progress in Pediatrics: Osteodystrophia Fibrosa". Archives of Pediatrics & Adolescent Medicine 54 (4): 806. doi:10.1001/archpedi.1937.01980040110009.
- ↑ "Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females: report of five cases". N. Engl. J. Med. 216 (17): 727–746. 1937. doi:10.1056/NEJM193704292161701.
- ↑ Anderson, Sharon (January 2020). "Café au Lait Macules and Associated Genetic Syndromes". Journal of Pediatric Health Care 34 (1): 71–81. doi:10.1016/j.pedhc.2019.05.001. ISSN 1532-656X. PMID 31831114.
- ↑ 6.0 6.1 6.2 6.3 Boyce, Alison M.; Collins, Michael T. (2020-04-01). "Fibrous Dysplasia/McCune-Albright Syndrome: A Rare, Mosaic Disease of Gα s Activation". Endocrine Reviews 41 (2): 345–370. doi:10.1210/endrev/bnz011. ISSN 1945-7189. PMID 31673695.
- ↑ 7.0 7.1 Estrada, Andrea; Boyce, Alison M.; Brillante, Beth A.; Guthrie, Lori C.; Gafni, Rachel I.; Collins, Michael T. (November 2016). "Long-term Outcomes of Letrozole Treatment for Precocious Puberty in Girls with McCune-Albright Syndrome". European Journal of Endocrinology 175 (5): 477–483. doi:10.1530/EJE-16-0526. ISSN 0804-4643. PMID 27562402.
- ↑ 8.0 8.1 8.2 8.3 8.4 Boyce, Alison M; Collins, Michael T (1993). "Fibrous Dysplasia/McCune-Albright Syndrome". Fibrous Dysplasia/McCune-Albright Syndrome Gene Reviews. University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK274564/. Retrieved 6 January 2018.
- ↑ 9.0 9.1 Celi, Francesco S.; Coppotelli, Giuseppe; Chidakel, Aaron; Kelly, Marilyn; Brillante, Beth A.; Shawker, Thomas; Cherman, Natasha; Feuillan, Penelope P. et al. (June 2008). "The Role of Type 1 and Type 2 5′-Deiodinase in the Pathophysiology of the 3,5,3′-Triiodothyronine Toxicosis of McCune-Albright Syndrome". The Journal of Clinical Endocrinology and Metabolism 93 (6): 2383–2389. doi:10.1210/jc.2007-2237. ISSN 0021-972X. PMID 18349068.
- ↑ 10.0 10.1 Salenave, Sylvie; Boyce, Alison M.; Collins, Michael T.; Chanson, Philippe (June 2014). "Acromegaly and McCune-Albright Syndrome". The Journal of Clinical Endocrinology and Metabolism 99 (6): 1955–1969. doi:10.1210/jc.2013-3826. ISSN 0021-972X. PMID 24517150.
- ↑ Cutler, Carolee M.; Lee, Janice S.; Butman, John A.; FitzGibbon, Edmond J.; Kelly, Marilyn H.; Brillante, Beth A.; Feuillan, Penelope; Robey, Pamela G. et al. (November 2006). "Long-term outcome of optic nerve encasement and optic nerve decompression in patients with fibrous dysplasia: risk factors for blindness and safety of observation". Neurosurgery 59 (5): 1011–1017; discussion 1017–1018. doi:10.1227/01.NEU.0000254440.02736.E3. ISSN 1524-4040. PMID 17143235.
- ↑ Boyce, Alison M.; Brewer, Carmen; DeKlotz, Timothy R.; Zalewski, Christopher K.; King, Kelly A.; Collins, Michael T.; Kim, H. Jeffrey (1 February 2018). "Association of Hearing Loss and Otologic Outcomes With Fibrous Dysplasia". JAMA Otolaryngology–Head & Neck Surgery 144 (2): 102–107. doi:10.1001/jamaoto.2017.2407. ISSN 2168-619X. PMID 29192304.
- ↑ Brown, Rebecca J.; Kelly, Marilyn H.; Collins, Michael T. (April 2010). "Cushing Syndrome in the McCune-Albright Syndrome". The Journal of Clinical Endocrinology and Metabolism 95 (4): 1508–1515. doi:10.1210/jc.2009-2321. ISSN 0021-972X. PMID 20157193.
- ↑ "Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations". J. Clin. Endocrinol. Metab. 88 (9): 4413–7. September 2003. doi:10.1210/jc.2002-021642. PMID 12970318. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=12970318.
- ↑ Schwindinger, W F; Francomano, C A; Levine, M A (June 1992). "Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome.". Proceedings of the National Academy of Sciences 89 (11): 5152–5156. doi:10.1073/pnas.89.11.5152. ISSN 0027-8424. PMID 1594625.
- ↑ Happle, R. (1986). "The McCune-Alrbight syndrome: a lethal gene surviving by mosaicism.". Clinical Genetics 29 (4): 321–324. doi:10.1111/j.1399-0004.1986.tb01261.x. PMID 3720010.
- ↑ 17.0 17.1 17.2 17.3 Dumitrescu, Claudia E.; Collins, Michael T. (19 May 2008). "McCune-Albright syndrome". Orphanet Journal of Rare Diseases 3 (1): 12. doi:10.1186/1750-1172-3-12. PMID 18489744.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 18.7 Javaid, Muhammad Kassim; Boyce, Alison; Appelman-Dijkstra, Natasha; Ong, Juling; Defabianis, Patrizia; Offiah, Amaka; Arundel, Paul; Shaw, Nick et al. (13 June 2019). "Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium". Orphanet Journal of Rare Diseases 14 (1): 139. doi:10.1186/s13023-019-1102-9. ISSN 1750-1172. PMID 31196103.
- ↑ Kushchayeva, Yevgeniya S.; Kushchayev, Sergiy V.; Glushko, Tetiana Y.; Tella, Sri Harsha; Teytelboym, Oleg M.; Collins, Michael T.; Boyce, Alison M. (December 2018). "Fibrous dysplasia for radiologists: beyond ground glass bone matrix". Insights into Imaging 9 (6): 1035–1056. doi:10.1007/s13244-018-0666-6. ISSN 1869-4101. PMID 30484079.
- ↑ Florenzano, Pablo; Pan, Kristen S.; Brown, Sydney M.; Paul, Scott M.; Kushner, Harvey; Guthrie, Lori C.; de Castro, Luis Fernandez; Collins, Michael T. et al. (April 2019). "Age-Related Changes and Effects of Bisphosphonates on Bone Turnover and Disease Progression in Fibrous Dysplasia of Bone". Journal of Bone and Mineral Research 34 (4): 653–660. doi:10.1002/jbmr.3649. ISSN 1523-4681. PMID 30645769.
- ↑ Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, Bassim C, Cherman N, Ellsworth M, Kasa-Vubu JZ, Farley FA, Molinolo AA, Bhattacharyya N, Collins MT (2012). "Denosumab treatment for fibrous dysplasia". J Bone Miner Res 27 (7): 1462–70. doi:10.1002/jbmr.1603. PMID 22431375.
- ↑ 22.0 22.1 Salenave, Sylvie; Boyce, Alison M.; Collins, Michael T.; Chanson, Philippe (June 2014). "Acromegaly and McCune-Albright syndrome". The Journal of Clinical Endocrinology and Metabolism 99 (6): 1955–1969. doi:10.1210/jc.2013-3826. ISSN 1945-7197. PMID 24517150.
- ↑ 23.0 23.1 Boyce, Alison M.; Chong, William H.; Shawker, Thomas H.; Pinto, Peter A.; Linehan, W. Marsten; Bhattacharryya, Nisan; Merino, Maria J.; Singer, Frederick R. et al. (September 2012). "Characterization and management of testicular pathology in McCune-Albright syndrome". The Journal of Clinical Endocrinology and Metabolism 97 (9): E1782–1790. doi:10.1210/jc.2012-1791. ISSN 1945-7197. PMID 22745241.
- ↑ Tessaris, D.; Corrias, A.; Matarazzo, P.; De Sanctis, L.; Wasniewska, M.; Messina, M. F.; Vigone, M. C.; Lala, R. (2012). "Thyroid abnormalities in children and adolescents with McCune-Albright syndrome". Hormone Research in Paediatrics 78 (3): 151–157. doi:10.1159/000342641. ISSN 1663-2826. PMID 23006743.
- ↑ "McCune-Albright syndrome". https://ghr.nlm.nih.gov/condition/mccune-albright-syndrome.
External links
- GeneReviews entry for fibrous dysplasia/McCune–Albright Syndrome
| Classification | |
|---|---|
| External resources |
 |