Chemistry:Calcitriol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rocaltrol, Calcijex, Decostriol, others |
| Other names | 1,25-dihydroxycholecalciferol, 1alpha,25-dihydroxyvitamin D3, 1,25-dihydroxyvitamin D3, 1α,25-(OH)2D3, 1,25(OH)2D[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682335 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99.9% |
| Metabolism | Kidney |
| Elimination half-life | 5–8 hours (adults), 27 hours (children) |
| Excretion | Faeces (50%), urine (16%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
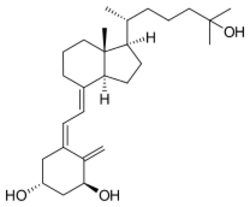
| Formula | C27H44O3 |
| Molar mass | 416.646 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Calcitriol is the active form of vitamin D, normally made in the kidney.[3][4][5] It is also known as 1,25-dihydroxycholecalciferol. It is a hormone which binds to and activates the vitamin D receptor in the nucleus of the cell, which then increases the expression of many genes.[6] Calcitriol increases blood calcium (Ca2+) mainly by increasing the uptake of calcium from the intestines.[2]
It can be given as a medication for the treatment of low blood calcium and hyperparathyroidism due to kidney disease, low blood calcium due to hypoparathyroidism, osteoporosis, osteomalacia, and familial hypophosphatemia,[2][7] and can be taken by mouth or by injection into a vein.[2] Excessive amounts or intake can result in weakness, headache, nausea, constipation, urinary tract infections, and abdominal pain.[2][7] Serious side effects may include high blood calcium and anaphylaxis.[2] Regular blood tests are recommended after the medication is started and when the dose is changed.[7]
Calcitriol was identified as the active form of vitamin D in 1971 and the drug was approved for medical use in the United States in 1978.[2] It is available as a generic medication.[7] In 2021, it was the 258th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[8][9] It is on the World Health Organization's List of Essential Medicines.[10]
Medical use
Calcitriol is prescribed for:[11]
- Treatment of hypocalcaemia – hypoparathyroidism, osteomalacia (adults), rickets (infants, children), renal osteodystrophy, chronic kidney disease
- Treatment of osteoporosis
- Prevention of corticosteroid-induced osteoporosis
Calcitriol has been used in an ointment for the treatment of psoriasis,[12] although the vitamin D analogue calcipotriol (calcipotriene) is more commonly used.[13] Calcitriol has also been given by mouth for the treatment of psoriasis[14] and psoriatic arthritis.[15] Research on the noncalcemic actions of calcitriol and other VDR-ligand analogs and their possible therapeutic applications has been reviewed.[16]
Adverse effects
The main adverse drug reaction associated with calcitriol therapy is hypercalcemia – early symptoms include: nausea, vomiting, constipation, anorexia, apathy, headache, thirst, pruritus, sweating, and/or polyuria. Compared to other vitamin D compounds in clinical use (cholecalciferol, ergocalciferol), calcitriol has a higher risk of inducing hypercalcemia. However, such episodes may be shorter and easier to treat due to its relatively short half-life.[11]
High calcitriol levels may also be seen in human disease states in patients not on supplementation. In someone with hypercalcaemia and high calcitriol levels, low intact parathyroid hormone levels are usually present.
The major conditions with hypercalcaemia due to elevated calcitriol levels are lymphoma, tuberculosis and sarcoidosis where excess production occurs due to ectopic 25(OH)D-1-hydroxylase (CYP27B1) expressed in macrophages.[17] Other conditions producing similar findings including:
- Fungal infections; Pneumocystis jiroveci, histoplasmosis, coccidioidomycosis, paracoccidioidomycosis, candidiasis
- Other granulomatous conditions; PR3+ vasculitis, Crohn's disease, acute granulomatous pneumonia, talc granuloma, silicone-induced granuloma, BCG-associated, granulomatous hepatitis, paraffin-associated granuloma
- Genetic conditions; Williams syndrome, pseudoxanthoma elasticum, CYP24A1 mutation (adult / infantile), SLC34A1 mutation
- Miscellaneous; mycobacterium avium, leprosy, lipoid pneumonia, cat scratch fever, berylliosis
Some plants contain glycosides of 1,25-dihydroxycholecalciferol. Consumption of these glycosides by grazing animals leads to vitamin D toxicity, resulting in calcinosis, the deposition of excessive calcium in soft tissues. Three rangeland plants, Cestrum diurnum, Solanum malacoxylon, and Trisetum flavescens are known to contain these glycosides. Of these, only C. diurnum is found in the U.S., mainly in Florida.[18]
Mechanism of action
Calcitriol increases blood calcium levels ([Ca2+]) by:
- Promoting absorption of dietary calcium from the gastrointestinal tract.
- Increasing renal tubular reabsorption of calcium, thus reducing the loss of calcium in the urine.
- Stimulating release of calcium from bone. For this it acts on the specific type of bone cells referred to as osteoblasts, causing them to release RANKL, which in turn activates osteoclasts.[19]
Calcitriol acts in concert with parathyroid hormone (PTH) in all three of these roles. For instance, PTH also indirectly stimulates osteoclasts. However, the main effect of PTH is to increase the rate at which the kidneys excrete inorganic phosphate (Pi), the counterion of Ca2+. The resulting decrease in serum phosphate causes hydroxyapatite (Ca5(PO4)3OH) to dissolve out of bone, thus increasing serum calcium. PTH also stimulates the production of calcitriol (see below).[20]
Many of the effects of calcitriol are mediated by its interaction with the calcitriol receptor, also called the vitamin D receptor or VDR.[21] For instance, the unbound inactive form of the calcitriol receptor in intestinal epithelial cells resides in the cytoplasm. When calcitriol binds to the receptor, the ligand-receptor complex translocates to the cell nucleus, where it acts as a transcription factor promoting the expression of a gene encoding a calcium binding protein. The levels of the calcium binding protein increase enabling the cells to actively transport more calcium (Ca2+) from the intestine across the intestinal mucosa into the blood.[20] Alternative, non-genomic pathways may be mediated through either PDIA3 or VDR.[22]
The maintenance of electroneutrality requires that the transport of Ca2+ ions catalyzed by the intestinal epithelial cells be accompanied by counterions, primarily inorganic phosphate. Thus calcitriol also stimulates the intestinal absorption of phosphate.[20]
The observation that calcitriol stimulates the release of calcium from bone seems contradictory, given that sufficient levels of serum calcitriol generally prevent overall loss of calcium from bone. It is believed that the increased levels of serum calcium resulting from calcitriol-stimulated intestinal uptake causes bone to take up more calcium than it loses by hormonal stimulation of osteoclasts.[20] Only when there are conditions, such as dietary calcium deficiency or defects in intestinal transport, which result in a reduction of serum calcium does an overall loss of calcium from bone occur.
Calcitriol also inhibits the release of calcitonin,[23] a hormone which reduces blood calcium primarily by inhibiting calcium release from bone.[20]
Biosynthesis and its regulation

Calcitriol is produced in the cells of the proximal tubule of the nephron in the kidneys by the action of 25-hydroxyvitamin D3 1-alpha-hydroxylase, a mitochondrial oxygenase and an enzyme which catalyzes the hydroxylation of 25-hydroxycholecalciferol (calcifediol) in the 1-alpha position.
The activity of this enzyme is stimulated by PTH. This is an important control point in Ca2+ homeostasis.[20] Additional effects on the production of calcitriol include an increase by prolactin, a hormone which stimulates lactogenesis (the formation of milk in mammary glands), a process which requires large amounts of calcium.[24] Activity is also decreased by high levels of serum phosphate and by an increase in the production of the hormone FGF23 by osteocyte cells in bone.[25]
Calcitriol is also produced outside the kidney in small amounts by many other tissues including placenta and activated macrophages.[26]
When the drug alfacalcidol is used, 25-hydroxylation in the liver produces calcitriol as the active metabolite. This will produce greater effects than other vitamin D precursors in patients with kidney disease who have loss of the renal 1-alpha-hydroxylase.[27]
Interactive pathway map
Metabolism
The halflife of calcitriol in the body is measured in hours, unlike its precursor calcifediol, whose halflife is measured in weeks.[28] Calcitriol is inactivated by further hydroxylation to form 1,24,25-trihydroxyvitamin D, calcitroic acid. This occurs through the action of the CYP24A1 24-hydroxylase.[29] Calcitroic acid is more soluble in water and is excreted in bile and urine.
History
It was first identified in 1971 by Michael F. Holick working in the laboratory of Hector DeLuca,[30][31] and also by Tony Norman and colleagues.[32]
Names
Calcitriol refers specifically to 1,25-dihydroxycholecalciferol. Because cholecalciferol already has one hydroxyl group, only two (1,25) are further specified in this nomenclature, but in fact there are three (1,3,25-triol), as indicated by the name calcitriol. The 1-hydroxy group is in the alpha position, and this may be specified in the name, for instance in the abbreviation 1α,25-(OH)2D3.[1]
Calcitriol is, strictly, the 1-hydroxylation product of calcifediol (25-OH vitamin D3), derived from cholecalciferol (vitamin D3), rather than the product of hydroxylations of ergocalciferol (vitamin D2).[1] 1α,25-Dihydroxyergocalciferol (ercalcitriol) should be used for the vitamin D2 product.[1] However, the terminology of 1,25-dihydroxyvitamin D, or 1,25(OH)2D, is often used to refer to both types of active forms of vitamin D. Indeed, both bind to the vitamin D receptor and produce biological effects.[33] In clinical use, the differences are unlikely to have major importance.[27]
Calcitriol is marketed as a pharmaceutical for medical use under various trade names including Rocaltrol (Roche), Calcijex (Abbott), Decostriol (Mibe, Jesalis), Vectical (Galderma), and Rolsical (Sun Pharma).
References
- ↑ 1.0 1.1 1.2 1.3 "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN): Nomenclature of vitamin D. Recommendations 1981". European Journal of Biochemistry 124 (2): 223–227. May 1982. doi:10.1111/j.1432-1033.1982.tb06581.x. PMID 7094913.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Calcitriol Monograph for Professionals" (in en). American Society of Health-System Pharmacists. https://www.drugs.com/monograph/calcitriol.html.
- ↑ "Vitamin D, disease and therapeutic opportunities". Nature Reviews. Drug Discovery 9 (12): 941–955. December 2010. doi:10.1038/nrd3318. PMID 21119732.
- ↑ (in en) Encyclopedia of Endocrine Diseases. Academic Press. 2018. p. 344. ISBN 9780128122006. https://books.google.com/books?id=nVh7DwAAQBAJ&pg=RA4-PA344.
- ↑ "Office of Dietary Supplements - Vitamin D" (in en). 9 October 2020. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/.
- ↑ "From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health". The American Journal of Clinical Nutrition 88 (2): 491S–499S. August 2008. doi:10.1093/ajcn/88.2.491S. PMID 18689389.
- ↑ 7.0 7.1 7.2 7.3 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 1050–1051. ISBN 9780857113382.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Calcitriol - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Calcitriol.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ 11.0 11.1 Rossi, Simone (2006). Australian Medicines Handbook. Adelaide. ISBN 978-0-9757919-2-9.
- ↑ "Efficacy and safety of topical calcitriol 3 microg/g ointment, a new topical therapy for chronic plaque psoriasis". Journal of Drugs in Dermatology 8 (8 Suppl): s9-16. August 2009. PMID 19702031.
- ↑ "Calcipotriene and betamethasone dipropionate for the topical treatment of plaque psoriasis". Expert Review of Clinical Pharmacology 9 (6): 789–797. June 2016. doi:10.1080/17512433.2016.1179574. PMID 27089906.
- ↑ "A novel approach for the evaluation and treatment of psoriasis. Oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis". Journal of the American Academy of Dermatology 19 (3): 516–528. September 1988. doi:10.1016/S0190-9622(88)70207-8. PMID 2459166.
- ↑ "Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: a pilot study". Arthritis and Rheumatism 33 (11): 1723–1727. November 1990. doi:10.1002/art.1780331117. PMID 2242069.
- ↑ "Noncalcemic actions of vitamin D receptor ligands". Endocrine Reviews 26 (5): 662–687. August 2005. doi:10.1210/er.2004-0002. PMID 15798098..
- ↑ "Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment". Endocrine Reviews 37 (5): 521–547. October 2016. doi:10.1210/er.2016-1070. PMID 27588937.
- ↑ "Calcinogenic Glycosides". Cornell Department of Animal Science. http://poisonousplants.ansci.cornell.edu/toxicagents/calglyco.html.
- ↑ "Bone and Mineral Metabolism in Health and Disease". Harrison's Principles of Internal Medicine (17th ed.). McGraw-Hill. 2008. ISBN 978-0-07-159991-7. http://www.accessmedicine.com/content.aspx?aID=2882031.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 "Biomolecules, mechanisms of enzyme action, and metabolism". Biochemistry. 1 (3rd ed.). Wiley. 2004. pp. 663–4. ISBN 978-0-471-25090-6. https://archive.org/details/biochemistry00voet_1/page/663.
- ↑ "Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects". Physiological Reviews 96 (1): 365–408. January 2016. doi:10.1152/physrev.00014.2015. PMID 26681795.
- ↑ "The Non-Genomic Actions of Vitamin D". Nutrients 8 (3): 135. March 2016. doi:10.3390/nu8030135. PMID 26950144.
- ↑ "Down-regulation of calcitonin gene transcription by vitamin D requires two widely separated enhancer sequences". Molecular Endocrinology 7 (8): 999–1008. August 1993. doi:10.1210/mend.7.8.8232320. PMID 8232320.
- ↑ "Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin". Endocrinology 151 (7): 2974–2984. July 2010. doi:10.1210/en.2010-0033. PMID 20463051.
- ↑ "FGF23 as a calciotropic hormone". F1000Research 4: 1472. 2015. doi:10.12688/f1000research.7189.1. PMID 27081473.
- ↑ "Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase". Archives of Biochemistry and Biophysics 523 (1): 95–102. July 2012. doi:10.1016/j.abb.2012.02.016. PMID 22446158.
- ↑ 27.0 27.1 "Vitamin D metabolites and/or analogs: which D for which patient?". Current Vascular Pharmacology 12 (2): 339–349. March 2014. doi:10.2174/15701611113119990024. PMID 23713876.
- ↑ "Indications on the use of vitamin D and vitamin D metabolites in clinical phenotypes". Clinical Cases in Mineral and Bone Metabolism 7 (3): 243–250. September 2010. PMID 22460535.
- ↑ "Cytochrome P450-mediated metabolism of vitamin D". Journal of Lipid Research 55 (1): 13–31. January 2014. doi:10.1194/jlr.R031534. PMID 23564710.
- ↑ "Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine". Biochemistry 10 (14): 2799–2804. July 1971. doi:10.1021/bi00790a023. PMID 4326883.
- ↑ "Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine". Proceedings of the National Academy of Sciences of the United States of America 68 (4): 803–804. April 1971. doi:10.1073/pnas.68.4.803. PMID 4323790. Bibcode: 1971PNAS...68..803H.
- ↑ "1,25-dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine". Science 173 (3991): 51–54. July 1971. doi:10.1126/science.173.3991.51. PMID 4325863. Bibcode: 1971Sci...173...51N.
- ↑ "Vitamin D and 1,25(OH)2D regulation of T cells". Nutrients 7 (4): 3011–3021. April 2015. doi:10.3390/nu7043011. PMID 25912039.
 |
