Medicine:Scleroderma
| Scleroderma | |
|---|---|
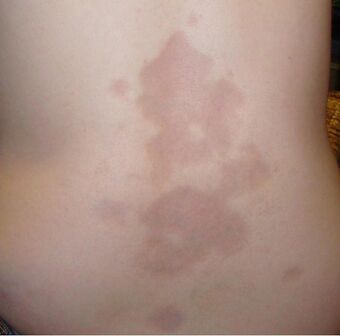 | |
| A type of localized scleroderma known as morphea | |
| Specialty | Rheumatology |
| Usual onset | Middle age[1] |
| Types | Localized, systemic scleroderma[2] |
| Causes | Unknown[2] |
| Risk factors | Family history, certain genetic factors, exposure to silica[3][4][5] |
| Diagnostic method | Based on symptoms, skin biopsy, blood tests[6] |
| Differential diagnosis | Mixed connective tissue disease, systemic lupus erythematosus, polymyositis, dermatomyositis[1] |
| Treatment | Supportive care[1] |
| Medication | Corticosteroids, methotrexate, non-steroidal anti-inflammatory drugs (NSAIDs)[2] |
| Prognosis | Localized: Normal life expectancy[7] Systemic: Decreased life expectancy[3] |
| Frequency | 3 per 100,000 per year (systemic)[3] |
Scleroderma is a group of autoimmune diseases that may result in changes to the skin, blood vessels, muscles, and internal organs.[2][6][8] The disease can be either localized to the skin or involve other organs, as well.[2] Symptoms may include areas of thickened skin, stiffness, feeling tired, and poor blood flow to the fingers or toes with cold exposure.[1] One form of the condition, known as CREST syndrome, classically results in calcium deposits, Raynaud's syndrome, esophageal problems, thickening of the skin of the fingers and toes, and areas of small, dilated blood vessels.[1]
The cause is unknown, but it may be due to an abnormal immune response.[2] Risk factors include family history, certain genetic factors, and exposure to silica.[3][4][5] The underlying mechanism involves the abnormal growth of connective tissue, which is believed to be the result of the immune system attacking healthy tissues.[6] Diagnosis is based on symptoms, supported by a skin biopsy or blood tests.[6]
While no cure is known, treatment may improve symptoms.[2] Medications used include corticosteroids, methotrexate, and non-steroidal anti-inflammatory drugs (NSAIDs).[2] Outcome depends on the extent of disease.[3] Those with localized disease generally have a normal life expectancy.[7] In those with systemic disease, life expectancy can be affected, and this varies based on subtype.[3] Death is often due to lung, gastrointestinal, or heart complications.[3]
About three per 100,000 people per year develop the systemic form.[3] The condition most often begins in middle age.[1] Women are more often affected than men.[1] Scleroderma symptoms were first described in 1753 by Carlo Curzio[9] and then well documented in 1842.[10] The term is from the Greek skleros meaning "hard" and derma meaning "skin".[11][12]
Signs and symptoms
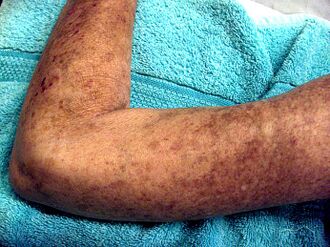

Potential signs and symptoms include:[13][14][15]
- Cardiovascular: Raynaud's phenomenon (is the presenting symptom in 30% of affected persons, occurs in 95% of affected individuals at some time during their illness); healed pitting ulcers on the fingertips; skin and mucosal telangiectasis; palpitations, irregular heart rate and fainting due to conduction abnormalities, hypertension, and congestive heart failure
- Digestive: gastroesophageal reflux disease, bloating, indigestion, loss of appetite, diarrhoea alternating with constipation, sicca syndrome and its complications, loosening of teeth, and hoarseness (due to acid reflux).
- Pulmonary: progressive worsening of shortness of breath, chest pain (due to pulmonary artery hypertension) and dry, persistent cough due to interstitial lung disease
- Musculoskeletal: joint, muscle aches, loss of joint range of motion, carpal tunnel syndrome, and muscle weakness
- Genitourinary: erectile dysfunction, dyspareunia, kidney problems, or kidney failure
- Other: facial pain due to trigeminal neuralgia, hand paresthesias, headache, stroke, fatigue, calcinosis, and weight loss
Cause
Scleroderma is caused by genetic and environmental factors.[4][5][16][17] Mutations in HLA genes seem to play a crucial role in the pathogenesis of some cases; likewise silica, aromatic and chlorinated solvents, ketones, trichloroethylene, welding fumes, and white spirits exposure seems to contribute to the condition in a small proportion of affected persons.[4][5][16][17][18]
Pathophysiology
Scleroderma is characterised by increased synthesis of collagen (leading to the sclerosis), damage to small blood vessels, activation of T lymphocytes, and production of altered connective tissue.[19] Its proposed pathogenesis is the following:[20][21][22][23][24]
- It begins with an inciting event at the level of the vasculature, probably the endothelium. The inciting event is yet to be elucidated, but may be a viral agent, oxidative stress, or autoimmune. Endothelial cell damage and apoptosis ensue, leading to the vascular leakiness that manifests in early clinical stages as tissue oedema. At this stage, it is predominantly a Th1- and Th17-mediated disease.
- After this, the vasculature is further compromised by impaired angiogenesis and impaired vasculogenesis (fewer endothelial progenitor cells), likely related to the presence of antiendothelinal cell antibodies (AECA). Despite this impaired angiogenesis, elevated levels of pro-angiogenic growth factors such as PDGF and VEGF is often seen in persons with the condition. The balance of vasodilation and vasoconstriction becomes askew, and the net result is vasoconstriction. The damaged endothelium then serves as a point of origin for blood-clot formation and further contributes to ischaemia-reperfusion injury and the generation of reactive oxygen species. These later stages are characterised by Th2 polarity.
- The damaged endothelium upregulates adhesion molecules and chemokines to attract leucocytes, which enables the development of innate and adaptive immune responses, including loss of tolerance to various oxidised antigens, which includes topoisomerase I. B cells mature into plasma cells, which furthers the autoimmune component of the condition. T cells differentiate into subsets, including Th2 cells, which play a vital role in tissue fibrosis. Anti–topoisomerase 1 antibodies, in turn, stimulate type I interferon production.
- Fibroblasts are recruited and activated by multiple cytokines and growth factors to generate myofibroblasts. Dysregulated transforming growth factor β (TGF-β) signalling in fibroblasts and myofibroblasts has been observed in multiple studies of scleroderma-affected individuals. Activation of fibroblasts and myofibroblasts leads to excessive deposition of collagen and other related proteins, leading to fibrosis. B cells are implicated in this stage, IL-6 and TGF-β produced by the B cells decrease collagen degradation and increase extracellular matrix production. Endothelin signalling is implicated in the pathophysiology of fibrosis.[25]
Vitamin D is implicated in the pathophysiology of the disease. An inverse correlation between plasma levels of vitamin D and scleroderma severity has been noted, and vitamin D is known to play a crucial role in regulating (usually suppressing) the actions of the immune system.[26]
Diagnosis
Typical scleroderma is classically defined as symmetrical skin thickening, with about 70% of cases also presenting with Raynaud's phenomenon, nail-fold capillary changes, and antinuclear antibodies. Affected individuals may experience systemic organ involvement. No single test for scleroderma works all of the time, hence diagnosis is often a matter of exclusion. Atypical scleroderma may show any variation of these changes without skin changes or with finger swelling only.[27]
Laboratory testing can show antitopoisomerase antibodies, like anti-scl70 (causing a diffuse systemic form), or anticentromere antibodies (causing a limited systemic form and the CREST syndrome). Other autoantibodies can be seen, such as anti-U3 or anti-RNA polymerase.[28] Antidouble-stranded DNA autoantibodies are likely to be present in serum.[citation needed]
Differential
Diseases that are often in the differential include:[29]
- Eosinophilia is a condition in which too many eosinophils (a type of immune cell that attacks parasites and is involved in certain allergic reactions) are present in the blood.
- Eosinophilia-myalgia syndrome is a form of eosinophilia caused by L-tryptophan supplements.
- Eosinophilic fasciitis affects the connective tissue surrounding skeletal muscles, bones, blood vessels, and nerves in the arms and legs.
- Graft-versus-host disease is an autoimmune condition that occurs as a result of bone-marrow transplants in which the immune cells from the transplanted bone marrow attack the host's body.
- Mycosis fungoides is a type of cutaneous T cell lymphoma, a rare cancer that causes rashes all over the body.
- Nephrogenic systemic fibrosis is a condition usually caused by kidney failure that results in fibrosis (thickening) of the tissues.
- Primary biliary cirrhosis is an autoimmune disease of the liver.
- Primary pulmonary hypertension
- Complex regional pain syndrome
Classification
Scleroderma is characterised by the appearance of circumscribed or diffuse, hard, smooth, ivory-colored areas that are immobile and which give the appearance of hidebound skin, a disease occurring in both localised and systemic forms:[30]
- Localised scleroderma
- Localised morphea
- Morphea-lichen sclerosus et atrophicus overlap
- Generalised morphea
- Atrophoderma of Pasini and Pierini
- Pansclerotic morphea
- Morphea profunda
- Linear scleroderma
- Systemic scleroderma
- CREST syndrome
- Progressive systemic sclerosis
Treatment
No cure for scleroderma is known, although relief of symptoms is often achieved; these include treatment of:[13][31]
- Raynaud's phenomenon with vasodilators such as calcium channel blockers, alpha blockers, serotonin receptor antagonists, angiotensin II receptor inhibitors, statins, local nitrates or iloprost
- Digital ulcers with phosphodiesterase 5 inhibitors (e.g., sildenafil) or iloprost
- Prevention of new digital ulcers with bosentan
- Malnutrition, secondary to intestinal flora overgrowth with tetracycline antibiotics such as tetracycline
- Interstitial lung disease with cyclophosphamide, azathioprine with or without corticosteroids
- Pulmonary arterial hypertension with endothelin receptor antagonists, phosphodiesterase 5 inhibitors, and prostanoids
- Gastrooesophageal reflux disease with antacids or prokinetics
- Kidney crises with angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists
Systemic disease-modifying treatment with immunosuppressants is often used.[16][32][33][34][35][36] Immunosuppressants used in its treatment include azathioprine, methotrexate, cyclophosphamide, mycophenolate, intravenous immunoglobulin, rituximab, sirolimus, alefacept, and the tyrosine kinase inhibitors, imatinib, nilotinib, and dasatinib.[16][31][32][33][34][35][36][37]
Experimental therapies under investigation include endothelin receptor antagonists, tyrosine kinase inhibitors, beta-glycan peptides, halofuginone, basiliximab, alemtuzumab, abatacept, and haematopoietic stem cell transplantation.[38][39]
| Immunomodulatory agents in the treatment of scleroderma | ||||
|---|---|---|---|---|
| INN | Mechanism of action[40][41] | Route of administration[40] | Pregnancy category[40][42] | Major toxicities[40] |
| Alefacept | Monoclonal antibody to inhibit T lymphocyte activation by binding to CD2 portion of human leukocyte function antigen-3. | IM | B (US) | Malignancies, injection site reactions, blood clots, lymphopenia, hepatotoxicity and infections. |
| Azathioprine | Purine analogue that inhibits lymphocyte proliferation via conversion to mercaptopurine | PO, IV | D (Au) | Myelosuppression and rarely malignancy, hepatitis, infection, hepatic sinusoidal obstruction syndrome and hypersensitivity reactions. |
| Cyclophosphamide | Nitrogen mustard that cross-links DNA base pairs, leading to breakages and triggering apoptosis in haematopoietic cells. | PO, IV | D (Au) | Vomiting, myelosuppression, haemorrhagic cystitis and rarely heart failure, pulmonary fibrosis, hepatic sinusoidal obstruction syndrome, malignancy and SIADH |
| Dasatinib | Tyrosine kinase inhibitor against various proangiogenic growth factors (including PDGF and VEGF). | PO | D (Au) | Fluid retention, myelosuppression, haemorrhage, infections, pulmonary hypertension, electrolyte anomalies and uncommonly hepatotoxicity, heart dysfunction/failure, myocardial infarction, QT interval prolongation, renal failure and hypersensitivity. |
| Imatinib | As above | PO | D (Au) | As above and rarely: GI perforation, avascular necrosis and rhabdomyolysis |
| Immunoglobulin | Immunoglobulin, modulates the immune system. | IV | N/A | Varies |
| Methotrexate | Antifolate; inhibits dihydrofolate reductase. | PO, IV, IM, SC, IT | D (Au) | Myeosuppression, pulmonary toxicity, hepatotoxicity, neurotoxicity and rarely kidney failure, hypersensitivity reactions, skin and bone necrosis, and osteoporosis |
| Mycophenolate | Inosine monophosphate dehydrogenase inhibitor, leading to reduced purine biosynthesis in lymphocytes. | PO, IV | D (Au) | Myelosuppression, blood clots, less commonly GI perforation/haemorrhage and rarely pancreatitis, hepatitis, aplastic anaemia and pure red cell aplasia. |
| Nilotinib | As per dasatinib | PO | D (Au) | As per imatinib |
| Rituximab | Monoclonal antibody against CD20, which is expressed on B lymphocytes | IV | C (Au) | Infusion-related reactions, infection, neutropenia, reduced immunoglobulin levels, arrhythmias, less commonly anaemia, thrombocytopenia, angina, myocardial infarction, heart failure, and rarely haemolytic anaemia, aplastic anaemia, serum sickness, severe skin conditions, pulmonary infiltrates, pneumonitis, cranial neuropathy (vision or hearing loss) and progressive multifocal leucoencephalopathy. |
| Sirolimus | mTOR inhibitor, thereby reducing cytokine-induced lymphocyte proliferation. | PO | C (Au) | Neutropenia, hypokalaemia, interstitial lung disease, pericardial effusion, pleural effusion, less commonly pulmonary haemorrhage, nephrotic syndrome, and rarely hepatotoxicity and lymphoedema. |
| PO = Oral. IV = Intravenous. IM = Intramuscular. SC = Subcutaneous. IT = Intrathecal. The preferred pregnancy category, above, is Australian, if available. If unavailable, an American one is substituted. | ||||
Prognosis
As of 2012[update], the five-year survival rate for systemic scleroderma was about 85%, whereas the 10-year survival rate was just under 70%.[43] This varies according to the subtype; while localized scleroderma rarely results in death, the systemic form can, and the diffuse systemic form carries a worse prognosis than the limited form. The major scleroderma-related causes of death are: pulmonary hypertension, pulmonary fibrosis, and scleroderma renal crisis.[28] People with scleroderma are also at a heightened risk for contracting cancers (especially liver, lung, haematologic, and bladder cancers), and perhaps, cardiovascular disease.[44][45][46][47][48][49][excessive citations]
According to a study of an Australian cohort, between 1985 and 2015, the average life expectancy of a person with scleroderma increased from 66 years to 74 years (around 8 years less than the average Australian life expectancy of 82 years).[50]
Epidemiology
Scleroderma most commonly first presents between the ages of 20 and 50 years, although any age group can be affected.[13][28] Women are four to nine times more likely to develop scleroderma than men.[28]
This disease is found worldwide.[28] In the United States, prevalence is estimated at 240 per million and the annual incidence of scleroderma is 19 per million people.[28] Likewise in the United States, it is slightly more common in African Americans than in their white counterparts. Choctaw Native Americans are more likely than Americans of European descent to develop the type of scleroderma that affects internal organs.[28] In Germany , the prevalence is between 10 and 150 per million people, and the annual incidence is between three and 28 per million people.[43] In South Australia, the annual incidence is 23 per million people, and the prevalence 233 per million people.[51]
Pregnancy
Scleroderma in pregnancy is a complex situation; it increases the risk to both mother and child.[52] Overall, scleroderma is associated with reduced fetal weight for gestational age.[52] The treatment for scleroderma often includes known teratogens such as cyclophosphamide, methotrexate, mycophenolate, etc., so careful avoidance of such drugs during pregnancy is advised.[52] In these cases hydroxychloroquine and low-dose corticosteroids might be used for disease control.[52]
See also
- Congenital fascial dystrophy
- Chi Chi DeVayne, (developed scleroderma in the years leading up to her death)
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Scleroderma". 2007. https://rarediseases.org/rare-diseases/scleroderma/.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Scleroderma" (in en). 2017. https://rarediseases.info.nih.gov/diseases/10308/scleroderma.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Jameson, Larry (2018). "Chapter 353". Harrison's Principles of Internal Medicine (20th ed.). McGraw Hill.
- ↑ 4.0 4.1 4.2 4.3 "Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers". Current Opinion in Rheumatology 24 (2): 165–70. March 2012. doi:10.1097/BOR.0b013e32834ff2e8. PMID 22269658.
- ↑ 5.0 5.1 5.2 5.3 "The immune pathogenesis of scleroderma: context is everything". Current Rheumatology Reports 15 (1): 297. January 2013. doi:10.1007/s11926-012-0297-8. PMID 23288576.
- ↑ 6.0 6.1 6.2 6.3 "Handout on Health: Scleroderma". August 2016. https://www.niams.nih.gov/%5C/Health_Info/Scleroderma/default.asp.
- ↑ 7.0 7.1 Unsal, Erbil (2006) (in en). Current Opinions in Pediatric Rheumatology. Nova Publishers. p. 302. ISBN 9781594548710. https://books.google.com/books?id=Hbavi8a3NqgC&pg=PA302.
- ↑ Denton, Christopher P; Khanna, Dinesh (October 2017). "Systemic sclerosis". The Lancet 390 (10103): 1685–99. doi:10.1016/s0140-6736(17)30933-9. PMID 28413064.
- ↑ Mackay, Ian R.; Rose, Noel R. (2006) (in en). The Autoimmune Diseases. Academic Press. p. 369. ISBN 9780080454740. https://books.google.com/books?id=gqttnHEe3jsC&pg=PA369.
- ↑ Firestein, Gary S.; Kelley, William N.; Budd, Ralph C. (2012) (in en). Kelley's Textbook of Rheumatology. Elsevier Health Sciences. p. 1366. ISBN 978-1437717389. https://books.google.com/books?id=52pQyDrAkOoC&pg=PA1366.
- ↑ Harper, Douglas. "scleroderma". Online Etymology Dictionary. https://www.etymonline.com/?term=scleroderma.
- ↑ σκληρός, δέρμα. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- ↑ 13.0 13.1 13.2 Hajj-ali, RA (June 2013). "Systemic Sclerosis". Merck Manual Professional. Merck Sharp & Dohme Corp.. http://www.merckmanuals.com/professional/musculoskeletal_and_connective_tissue_disorders/autoimmune_rheumatic_disorders/systemic_sclerosis.html.
- ↑ Varga, J; Talavera, F; Goldberg, E et al., eds (15 February 2012). "Scleroderma Clinical Presentation". Medscape Reference. WebMD. http://emedicine.medscape.com/article/331864-clinical#showall.
- ↑ Longo, D; Fauci, A; Kasper, D; Hauser, S; Jameson, J; Loscalzo, J (2011). Harrison's Principles of Internal Medicine (18th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07174889-6.[page needed]
- ↑ 16.0 16.1 16.2 16.3 "Scleroderma – new aspects in pathogenesis and treatment". Best Practice & Research. Clinical Rheumatology 26 (1): 13–24. February 2012. doi:10.1016/j.berh.2012.01.011. PMID 22424190.
- ↑ 17.0 17.1 "Environmental risk factors in systemic sclerosis". Current Opinion in Rheumatology 25 (2): 179–83. March 2013. doi:10.1097/BOR.0b013e32835cfc2d. PMID 23287382.
- ↑ "Prospective study to evaluate the association between systemic sclerosis and occupational exposure and review of the literature". Autoimmunity Reviews 13 (2): 151–56. February 2014. doi:10.1016/j.autrev.2013.10.002. PMID 24129037.
- ↑ "Pathogenesis and treatment modalities of localized scleroderma". Medicina 46 (10): 649–56. 2010. doi:10.3390/medicina46100092. PMID 21393982. http://medicina.kmu.lt/1010/1010-01e.pdf.
- ↑ "The pathogenesis of systemic sclerosis". Annual Review of Pathology 6: 509–37. 2011. doi:10.1146/annurev-pathol-011110-130312. PMID 21090968.
- ↑ "Angiogenic cytokines and growth factors in systemic sclerosis". Autoimmunity Reviews 10 (10): 590–94. August 2011. doi:10.1016/j.autrev.2011.04.019. PMID 21549861.
- ↑ "Cellular players in angiogenesis during the course of systemic sclerosis". Autoimmunity Reviews 10 (10): 641–46. August 2011. doi:10.1016/j.autrev.2011.04.016. PMID 21549220.
- ↑ "B cells in systemic sclerosis: a possible target for therapy". Autoimmunity Reviews 10 (10): 624–30. August 2011. doi:10.1016/j.autrev.2011.04.013. PMID 21545850.
- ↑ "Scleroderma: from pathophysiology to novel therapeutic approaches". Experimental Dermatology 19 (5): 393–400. May 2010. doi:10.1111/j.1600-0625.2010.01082.x. PMID 20507361.
- ↑ "The role of endothelin-1 signaling in the fibrosis observed in systemic sclerosis". Pharmacological Research 63 (6): 502–03. June 2011. doi:10.1016/j.phrs.2011.01.011. PMID 21315153.
- ↑ "Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature". Autoimmunity Reviews 10 (8): 490–94. June 2011. doi:10.1016/j.autrev.2011.02.002. PMID 21320645.
- ↑ Varga, J; Talavera, F; Goldberg, E et al., eds (15 February 2012). "Scleroderma Workup". Medscape Reference. WebMD. http://emedicine.medscape.com/article/331864-workup#showall.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 Varga, J; Talavera, F; Goldberg, E et al., eds (15 February 2012). "Scleroderma". Medscape Reference. WebMD. http://emedicine.medscape.com/article/331864-overview#showall.
- ↑ Varga, J; Talavera, F; Goldberg, E et al., eds (15 February 2012). "Scleroderma Differential Diagnoses". Medscape Reference. WebMD. http://emedicine.medscape.com/article/331864-workup#showall.
- ↑ Elston, William D. James, Timothy G. Berger, Dirk M. (2006). Andrew's diseases of the skin: clinical dermatology (10 ed.). Philadelphia, PA: Saunders Elsevier. pp. 169–172. ISBN 978-0808923510. https://archive.org/details/andrewsdiseasess00mdwi_659.
- ↑ 31.0 31.1 "Treatment of systemic sclerosis complications: what to use when first-line treatment fails--a consensus of systemic sclerosis experts". Seminars in Arthritis and Rheumatism 42 (1): 42–55. August 2012. doi:10.1016/j.semarthrit.2012.01.003. PMID 22464314.
- ↑ 32.0 32.1 "Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies". Clinics in Dermatology 31 (4): 432–37. July–August 2013. doi:10.1016/j.clindermatol.2013.01.010. PMID 23806160.
- ↑ 33.0 33.1 "My approach to the treatment of scleroderma". Mayo Clinic Proceedings 88 (4): 377–93. April 2013. doi:10.1016/j.mayocp.2013.01.018. PMID 23541012.
- ↑ 34.0 34.1 "Recent advances in the diagnosis and treatment of systemic sclerosis". Polskie Archiwum Medycyny Wewnetrznej 123 (1–2): 51–58. 2013. PMID 23344666. http://pamw.pl/sites/default/files/PAMW_2013-1-2_Kowal.pdf.
- ↑ 35.0 35.1 "Morphogen pathways as molecular targets for the treatment of fibrosis in systemic sclerosis". Archives of Dermatological Research 305 (1): 1–8. January 2013. doi:10.1007/s00403-012-1304-7. PMID 23208311.
- ↑ 36.0 36.1 "Emerging targets for the treatment of scleroderma". Expert Opinion on Emerging Drugs 17 (2): 173–79. June 2012. doi:10.1517/14728214.2012.678833. PMID 22533795.
- ↑ "Immunotherapy of systemic sclerosis". Immunotherapy 2 (6): 863–78. November 2010. doi:10.2217/imt.10.69. PMID 21091117.
- ↑ "Pharmacotherapy of systemic sclerosis". Expert Opinion on Pharmacotherapy 11 (5): 789–806. April 2010. doi:10.1517/14656561003592177. PMID 20210685.
- ↑ "Innovative therapies for systemic sclerosis". Current Opinion in Rheumatology 22 (3): 264–72. May 2010. doi:10.1097/BOR.0b013e328337c3d6. PMID 20190640.
- ↑ 40.0 40.1 40.2 40.3 Rossi, S, ed (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.[page needed]
- ↑ Brunton, L; Chabner, B; Knollman, B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8.[page needed]
- ↑ "Medscape Multispecialty – Home page". WebMD. http://reference.medscape.com/medscapetoday.[full citation needed]
- ↑ 43.0 43.1 "Systemic sclerosis-dermatological aspects. Part 1: Pathogenesis, epidemiology, clinical findings". Journal der Deutschen Dermatologischen Gesellschaft 10 (10): 705–18; quiz 716. October 2012. doi:10.1111/j.1610-0387.2012.07999.x. PMID 22913330.
- ↑ "The Prevalence of Atherosclerosis in Those with Inflammatory Connective Tissue Disease by Race, Age, and Traditional Risk Factors". Scientific Reports 6: 20303. February 2016. doi:10.1038/srep20303. PMID 26842423. Bibcode: 2016NatSR...620303A.
- ↑ "Cardiovascular disease in systemic sclerosis--an emerging association?". Arthritis Research & Therapy 13 (4): 237. August 2011. doi:10.1186/ar3445. PMID 21888685.
- ↑ "Malignancies associated with systemic sclerosis". Autoimmunity Reviews 11 (12): 852–55. October 2012. doi:10.1016/j.autrev.2012.02.021. PMID 22410174.
- ↑ "Cancer and systemic sclerosis: novel insights into pathogenesis and clinical implications". Current Opinion in Rheumatology 23 (6): 530–35. November 2011. doi:10.1097/BOR.0b013e32834a5081. PMID 21825998.
- ↑ "Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies". Arthritis and Rheumatism 65 (7): 1913–21. July 2013. doi:10.1002/art.37969. PMID 23576072.
- ↑ "Systemic sclerosis (scleroderma) and cancer risk: systematic review and meta-analysis of observational studies". Rheumatology 52 (1): 143–54. January 2013. doi:10.1093/rheumatology/kes303. PMID 23175568.
- ↑ "Improving life expectancy of patients with scleroderma: results from the South Australian Scleroderma Register". Internal Medicine Journal 48 (8): 951–56. 23 March 2018. doi:10.1111/imj.13799. PMID 29573101.
- ↑ "Epidemiology of systemic sclerosis". Best Practice & Research. Clinical Rheumatology 24 (6): 857–69. December 2010. doi:10.1016/j.berh.2010.10.007. PMID 21665131.
- ↑ 52.0 52.1 52.2 52.3 "Pregnancy issues in scleroderma". Autoimmunity Reviews 11 (6–7): A515–19. May 2012. doi:10.1016/j.autrev.2011.11.021. PMID 22155199.
External links
- Handout on Health: Scleroderma – US National Institute of Arthritis and Musculoskeletal and Skin Diseases
| Classification | |
|---|---|
| External resources |
 |
