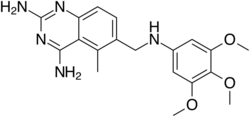
Chemistry:Trimetrexate
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a694019 |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | VD: 20-30 Liters |
| Metabolism | Oxidative O-demethylation, followed by conjugation with glucuronide or sulfate |
| Elimination half-life | 11 to 12 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H23N5O3 |
| Molar mass | 369.425 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Trimetrexate is a quinazoline derivative. It is a dihydrofolate reductase inhibitor.[1]
Uses
It has been used with leucovorin in treating pneumocystis pneumonia.[2]
It has been investigated for use in treating leiomyosarcoma.[3] It is a methotrexate (MTX) analog that is active against transport-deficient MTX-resistant tumor cells that overcome the acquired and natural resistance to methotrexate. Other uses include skin lymphoma. [4]
References
- ↑ "Metabolic disposition of trimetrexate, a nonclassical dihydrofolate reductase inhibitor, in rat and dog". Drug Metab. Dispos. 18 (6): 980–6. 1990. PMID 1981548. http://dmd.aspetjournals.org/cgi/pmidlookup?view=long&pmid=1981548.
- ↑ "Trimetrexate-leucovorin dosage evaluation study for treatment of Pneumocystis carinii pneumonia". J. Infect. Dis. 161 (1): 91–6. January 1990. doi:10.1093/infdis/161.1.91. PMID 2136905.
- ↑ "Trimetrexate in the treatment of recurrent or advanced leiomyosarcoma of the uterus: a phase II study of the Gynecologic Oncology Group". Gynecol. Oncol. 84 (1): 140–4. January 2002. doi:10.1006/gyno.2001.6482. PMID 11748990.
- ↑ Trimetrexate in relapsed T-cell lymphoma with skin involvement. J Clin Oncol. 2002 Jun 15;20(12):2876-80.
External links
 |

