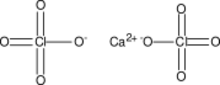
Chemistry:Calcium perchlorate
| |
| Names | |
|---|---|
| IUPAC name
Calcium perchlorate
| |
| Other names
Calcium perchlorate tetrahydrate, Calcium diperchlorate, Perchloric acid calcium salt (2:1), Calcium perchlorate, hydrated
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Ca(ClO 4) 2 | |
| Molar mass | 238.9792 g/mol |
| Appearance | White to yellow crystalline solid |
| Density | 2.651 g/cm3[1] |
| Melting point | 270 °C (518 °F; 543 K) [1] |
| 188.7 g/100 g[1] | |
| Solubility in acetone | 61.76 g/100 g |
| Solubility in ethyl acetate | 113.5 g/100 g |
| Solubility in ethanol | 166.2 g/100 g |
| Solubility in ethyl ether | 0.26 g/100 g |
| Hazards | |
| Main hazards | oxidiser |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H271 | |
| P210, P220, P221, P280, P283, P306+360, P370+378, P371+380+375, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcium perchlorate is classified as a metal perchlorate salt with the molecular formula Ca(ClO
4)
2. It is an inorganic compound that is a yellow-white crystalline solid in appearance. As a strong oxidizing agent, it reacts with reducing agents when heated to generate heat and products that may be gaseous (which will cause pressurization in closed containers). Calcium perchlorate has been categorized as having explosive reactivity. Ca(ClO
4)
2 is a common chemical on the soil of planet Mars, counting for almost 1% of the Martian dust, by weight.
Properties
Calcium perchlorate is a strong inorganic oxidizing agent, enhancing the combustion of other substances that can potentially lead to explosion. The perchlorate ion, ClO−
4, has a highly symmetrical tetrahedral structure that is strongly stabilized in solution by its low electron-donating proton-accepting power and its relatively low polarizability.
Eutectic system
Calcium perchlorate solution forms a simple eutectic system. The eutectic composition of the calcium perchlorate solution is 4.2 mol / 1000 g H
2O, very similar to the composition of closely related metal cation perchlorates of strontium and barium.[3][4]
Occurrences
Electrolyte conductance
Electrolyte conductance of Ca(ClO
4)
2 and double charged metal cations in the organic solvent acetonitrile has been tested.[5] The interest in metal cation perchlorate interactions with photosensitive ligands has increased due to the development of highly specific fluorescence indicators.
Production
Perchlorate salts are the product of a base and perchloric acid. Calcium perchlorate can be prepared through the heating of a mixture of calcium carbonate and ammonium perchlorate. Ammonium carbonate forms in the gaseous state, leaving behind a calcium perchlorate solid.[6][7]
Reactions
Water
Being very hygroscopic, calcium perchlorate is commonly seen in the presence of four water molecules, referred to as calcium perchlorate tetrahydrate Ca(ClO
4)
2 · 4H2O.[1]
Cyclic hydrogenphosphonates
A hybrid organic-inorganic molecule is formed using dioxazaphosphocanes, eight-membered cyclic hydrogenphosphonates and calcium. Calcium from the calcium perchlorate contributes to the structural integrity of the oligomeric molecule; the four calcium ions are bridged between four dioxazaphosphocane moieties.[8]
Human toxicity
Calcium perchlorate is slightly toxic to humans, by ingestion or inhalation of dust particles, or (less so) by skin contact.[9]
References
- ↑ 1.0 1.1 1.2 1.3 "Physical Constants of Inorganic Compounds", CRC Handbook of Chemistry and Physics (Taylor and Francis Group): p. 4-54, 2016, https://archive.org/details/CRCHandbookOfChemistryAndPhysics97thEdition2016/page/n771, retrieved June 4, 2023
- ↑ AMCP 706-187 Military Pyrotechnics - Properties of Materials. US Army Materiel Command. October 1963. p. 78. https://archive.org/details/AMCP706187MilitaryPyrotechnicsPropertiesOfMaterials/page/n93.
- ↑ Marion, G.M.; Catling, D.C.; Zahnle, K.J.; Claire, M.W. (June 2010). "Modeling aqueous perchlorate chemistries with applications to Mars" (in en). Icarus 207 (2): 675–685. doi:10.1016/j.icarus.2009.12.003. http://faculty.washington.edu/dcatling/Marion2010_PerchlorateFREZCHEM.pdf. Retrieved 5 November 2022.
- ↑ Pestova, O. N.; Myund, L. A.; Khripun, M. K.; Prigaro, A. V. (2005), "Polythermal Study of the Systems M(ClO4)2–H2O (M2+ = Mg2+, Ca2+, Sr2+, Ba2+)", Russian Journal of Applied Chemistry 78 (3): 409–413, doi:10.1007/s11167-005-0306-z
- ↑ Kalugin, Oleg N.; Agieienko, Vira N.; Otroshko, Natalya A. (January 2012), "Ion association and solvation in solutions of Mg2+, Ca2+, Sr2+, Ba2+ and Ni2+ perchlorates in acetonitrile: Conductometric study", Journal of Molecular Liquids 165: 78–86, doi:10.1016/j.molliq.2011.10.012
- ↑ "Calcium Perchlorate", ChemicalBook, http://www.chemicalbook.com/ProductChemicalPropertiesCB9181917_EN.htm, retrieved October 25, 2012
- ↑ "Perchlorates", Megalomania's Method of Making Perchlorates (Megalomania's Controversial Chem Lab), http://www.roguesci.org/megalomania/explo/perchlorates.html, retrieved June 4, 2023
- ↑ Sutra, Elsa; Lamandé, Lydia; Gornitzka, Heinz; Bellan, Jacques (2002), "A New Oligomeric Complex of Cyclic Hydrogenphosphonates with Calcium Perchlorate", European Journal of Inorganic Chemistry 2002 (10): 2727–2729, doi:10.1002/1099-0682(200210)2002:10<2727::AID-EJIC2727>3.0.CO;2-D
- ↑ "Calcium Perchlorate", Cameo Chemicals (Office of Response and Restoration, National Ocean Service), http://cameochemicals.noaa.gov/chemical/2787, retrieved October 25, 2012
 |


