Physics:Partition coefficient
In the physical sciences, a partition coefficient (P) or distribution coefficient (D) is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. This ratio is therefore a comparison of the solubilities of the solute in these two liquids. The partition coefficient generally refers to the concentration ratio of un-ionized species of compound, whereas the distribution coefficient refers to the concentration ratio of all species of the compound (ionized plus un-ionized).[1]
In the chemical and pharmaceutical sciences, both phases usually are solvents.[2] Most commonly, one of the solvents is water, while the second is hydrophobic, such as 1-octanol.[3] Hence the partition coefficient measures how hydrophilic ("water-loving") or hydrophobic ("water-fearing") a chemical substance is. Partition coefficients are useful in estimating the distribution of drugs within the body. Hydrophobic drugs with high octanol-water partition coefficients are mainly distributed to hydrophobic areas such as lipid bilayers of cells. Conversely, hydrophilic drugs (low octanol/water partition coefficients) are found primarily in aqueous regions such as blood serum.[4]
If one of the solvents is a gas and the other a liquid, a gas/liquid partition coefficient can be determined. For example, the blood/gas partition coefficient of a general anesthetic measures how easily the anesthetic passes from gas to blood.[5] Partition coefficients can also be defined when one of the phases is solid, for instance, when one phase is a molten metal and the second is a solid metal,[6] or when both phases are solids.[7] The partitioning of a substance into a solid results in a solid solution.
Partition coefficients can be measured experimentally in various ways (by shake-flask, HPLC, etc.) or estimated by calculation based on a variety of methods (fragment-based, atom-based, etc.).
If a substance is present as several chemical species in the partition system due to association or dissociation, each species is assigned its own Kow value. A related value, D, does not distinguish between different species, only indicating the concentration ratio of the substance between the two phases.[citation needed]
Nomenclature
Despite formal recommendation to the contrary, the term partition coefficient remains the predominantly used term in the scientific literature.[8][additional citation(s) needed]
In contrast, the IUPAC recommends that the title term no longer be used, rather, that it be replaced with more specific terms.[9] For example, partition constant, defined as
-
(KD)A = [A]org/ [A]aq,
()
where KD is the process equilibrium constant, [A] represents the concentration of solute A being tested, and "org" and "aq" refer to the organic and aqueous phases respectively. The IUPAC further recommends "partition ratio" for cases where transfer activity coefficients can be determined, and "distribution ratio" for the ratio of total analytical concentrations of a solute between phases, regardless of chemical form.[9]
Partition coefficient and log P

The partition coefficient, abbreviated P, is defined as a particular ratio of the concentrations of a solute between the two solvents (a biphase of liquid phases), specifically for un-ionized solutes, and the logarithm of the ratio is thus log P.[10]:275ff When one of the solvents is water and the other is a non-polar solvent, then the log P value is a measure of lipophilicity or hydrophobicity.[10]:275ff[11]:6 The defined precedent is for the lipophilic and hydrophilic phase types to always be in the numerator and denominator respectively; for example, in a biphasic system of n-octanol (hereafter simply "octanol") and water:
- [math]\displaystyle{ \log P_\text{oct/wat} = \log_{10}\left(\frac{\big[\text{solute}\big]_\text{octanol}^\text{un-ionized}}{\big[\text{solute}\big]_\text{water}^\text{un-ionized}}\right). }[/math]
To a first approximation, the non-polar phase in such experiments is usually dominated by the un-ionized form of the solute, which is electrically neutral, though this may not be true for the aqueous phase. To measure the partition coefficient of ionizable solutes, the pH of the aqueous phase is adjusted such that the predominant form of the compound in solution is the un-ionized, or its measurement at another pH of interest requires consideration of all species, un-ionized and ionized (see following).
A corresponding partition coefficient for ionizable compounds, abbreviated log P I, is derived for cases where there are dominant ionized forms of the molecule, such that one must consider partition of all forms, ionized and un-ionized, between the two phases (as well as the interaction of the two equilibria, partition and ionization).[11]:57ff,69f[12] M is used to indicate the number of ionized forms; for the I-th form (I = 1, 2, ... , M) the logarithm of the corresponding partition coefficient, [math]\displaystyle{ \log P_\text{oct/wat}^I }[/math], is defined in the same manner as for the un-ionized form. For instance, for an octanol–water partition, it is
- [math]\displaystyle{ \log\ P_\text{oct/wat}^\mathrm{I} = \log_{10}\left(\frac{\big[\text{solute}\big]_\text{octanol}^I}{\big[\text{solute}\big]_\text{water}^I}\right). }[/math]
To distinguish between this and the standard, un-ionized, partition coefficient, the un-ionized is often assigned the symbol log P0, such that the indexed [math]\displaystyle{ \log P_\text{oct/wat}^I }[/math] expression for ionized solutes becomes simply an extension of this, into the range of values I > 0.[citation needed]
Distribution coefficient and log D
The distribution coefficient, log D, is the ratio of the sum of the concentrations of all forms of the compound (ionized plus un-ionized) in each of the two phases, one essentially always aqueous; as such, it depends on the pH of the aqueous phase, and log D = log P for non-ionizable compounds at any pH.[13][14] For measurements of distribution coefficients, the pH of the aqueous phase is buffered to a specific value such that the pH is not significantly perturbed by the introduction of the compound. The value of each log D is then determined as the logarithm of a ratio—of the sum of the experimentally measured concentrations of the solute's various forms in one solvent, to the sum of such concentrations of its forms in the other solvent; it can be expressed as[10]:275–8
- [math]\displaystyle{ \log D_\text{oct/wat} = \log_{10}\left(\frac{\big[\text{solute}\big]_\text{octanol}^\text{ionized} + \big[\text{solute}\big]_\text{octanol}^\text{un-ionized}}{\big[\text{solute}\big]_\text{water}^\text{ionized} + \big[\text{solute}\big]_\text{water}^\text{un-ionized}}\right). }[/math]
In the above formula, the superscripts "ionized" each indicate the sum of concentrations of all ionized species in their respective phases. In addition, since log D is pH-dependent, the pH at which the log D was measured must be specified. In areas such as drug discovery—areas involving partition phenomena in biological systems such as the human body—the log D at the physiologic pH = 7.4 is of particular interest.[citation needed]
It is often convenient to express the log D in terms of PI, defined above (which includes P0 as state I = 0), thus covering both un-ionized and ionized species.[12] For example, in octanol–water:
- [math]\displaystyle{ \log D_\text{oct/wat} = \log_{10}\left(\sum_{I=0}^M f^I P_\text{oct/wat}^I \right), }[/math]
which sums the individual partition coefficients (not their logarithms), and where [math]\displaystyle{ f^I }[/math] indicates the pH-dependent mole fraction of the I-th form (of the solute) in the aqueous phase, and other variables are defined as previously.[12][verification needed]
Example partition coefficient data
The values for the octanol-water system in the following table are from the Dortmund Data Bank.[15][better source needed] They are sorted by the partition coefficient, smallest to largest (acetamide being hydrophilic, and 2,2',4,4',5-pentachlorobiphenyl lipophilic), and are presented with the temperature at which they were measured (which impacts the values).[citation needed]
| Component | log POW | T (°C) |
|---|---|---|
| Acetamide[16] | −1.16 | 25 |
| Methanol[17] | −0.81 | 19 |
| Formic acid[18] | −0.41 | 25 |
| Diethyl ether[17] | 0.83 | 20 |
| p-Dichlorobenzene[19] | 3.37 | 25 |
| Hexamethylbenzene[19] | 4.61 | 25 |
| 2,2',4,4',5-Pentachlorobiphenyl[20] | 6.41 | Ambient |
Values for other compounds may be found in a variety of available reviews and monographs.[2]:551ff[21][page needed][22]:1121ff[23][page needed][24] Critical discussions of the challenges of measurement of log P and related computation of its estimated values (see below) appear in several reviews.[11][24]
Applications
Pharmacology
A drug's distribution coefficient strongly affects how easily the drug can reach its intended target in the body, how strong an effect it will have once it reaches its target, and how long it will remain in the body in an active form.[25] Hence, the log P of a molecule is one criterion used in decision-making by medicinal chemists in pre-clinical drug discovery, for example, in the assessment of druglikeness of drug candidates.[26] Likewise, it is used to calculate lipophilic efficiency in evaluating the quality of research compounds, where the efficiency for a compound is defined as its potency, via measured values of pIC50 or pEC50, minus its value of log P.[27]
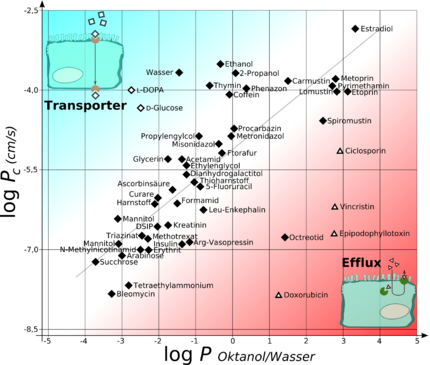
Pharmacokinetics
In the context of pharmacokinetics (how the body absorbs, metabolizes, and excretes a drug), the distribution coefficient has a strong influence on ADME properties of the drug. Hence the hydrophobicity of a compound (as measured by its distribution coefficient) is a major determinant of how drug-like it is. More specifically, for a drug to be orally absorbed, it normally must first pass through lipid bilayers in the intestinal epithelium (a process known as transcellular transport). For efficient transport, the drug must be hydrophobic enough to partition into the lipid bilayer, but not so hydrophobic, that once it is in the bilayer, it will not partition out again.[29][30] Likewise, hydrophobicity plays a major role in determining where drugs are distributed within the body after absorption and, as a consequence, in how rapidly they are metabolized and excreted.
Pharmacodynamics
In the context of pharmacodynamics (how the drug affects the body), the hydrophobic effect is the major driving force for the binding of drugs to their receptor targets.[31][32] On the other hand, hydrophobic drugs tend to be more toxic because they, in general, are retained longer, have a wider distribution within the body (e.g., intracellular), are somewhat less selective in their binding to proteins, and finally are often extensively metabolized. In some cases the metabolites may be chemically reactive. Hence it is advisable to make the drug as hydrophilic as possible while it still retains adequate binding affinity to the therapeutic protein target.[33] For cases where a drug reaches its target locations through passive mechanisms (i.e., diffusion through membranes), the ideal distribution coefficient for the drug is typically intermediate in value (neither too lipophilic, nor too hydrophilic); in cases where molecules reach their targets otherwise, no such generalization applies.[citation needed]
Environmental science
The hydrophobicity of a compound can give scientists an indication of how easily a compound might be taken up in groundwater to pollute waterways, and its toxicity to animals and aquatic life.[34] Partition coefficient can also be used to predict the mobility of radionuclides in groundwater.[35] In the field of hydrogeology, the octanol–water partition coefficient Kow is used to predict and model the migration of dissolved hydrophobic organic compounds in soil and groundwater.
Agrochemical research
Hydrophobic insecticides and herbicides tend to be more active. Hydrophobic agrochemicals in general have longer half-lives and therefore display increased risk of adverse environmental impact.[36]
Metallurgy
In metallurgy, the partition coefficient is an important factor in determining how different impurities are distributed between molten and solidified metal. It is a critical parameter for purification using zone melting, and determines how effectively an impurity can be removed using directional solidification, described by the Scheil equation.[6]
Consumer product development
Many other industries take into account distribution coefficients, for example in the formulation of make-up, topical ointments, dyes, hair colors and many other consumer products.[37]
Measurement
A number of methods of measuring distribution coefficients have been developed, including the shake-flask, separating funnel method, reverse-phase HPLC, and pH-metric techniques.[10]:280
Separating-funnel method
In this method the solid particles present into the two immiscible liquids can be easily separated by suspending those solid particles directly into these immiscible or somewhat miscible liquids.
Shake flask-type
The classical and most reliable method of log P determination is the shake-flask method, which consists of dissolving some of the solute in question in a volume of octanol and water, then measuring the concentration of the solute in each solvent.[38][39] The most common method of measuring the distribution of the solute is by UV/VIS spectroscopy.[38]
HPLC-based
A faster method of log P determination makes use of high-performance liquid chromatography. The log P of a solute can be determined by correlating its retention time with similar compounds with known log P values.[40]
An advantage of this method is that it is fast (5–20 minutes per sample). However, since the value of log P is determined by linear regression, several compounds with similar structures must have known log P values, and extrapolation from one chemical class to another—applying a regression equation derived from one chemical class to a second one—may not be reliable, since each chemical classes will have its characteristic regression parameters.[citation needed]
pH-metric
The pH-metric set of techniques determine lipophilicity pH profiles directly from a single acid-base titration in a two-phase water–organic-solvent system.[10]:280–4 Hence, a single experiment can be used to measure the logarithms of the partition coefficient (log P) giving the distribution of molecules that are primarily neutral in charge, as well as the distribution coefficient (log D) of all forms of the molecule over a pH range, e.g., between 2 and 12. The method does, however, require the separate determination of the pKa value(s) of the substance.
Electrochemical
Polarized liquid interfaces have been used to examine the thermodynamics and kinetics of the transfer of charged species from one phase to another. Two main methods exist. The first is ITIES, "interfaces between two immiscible electrolyte solutions".[41] The second is droplet experiments.[42] Here a reaction at a triple interface between a conductive solid, droplets of a redox active liquid phase and an electrolyte solution have been used to determine the energy required to transfer a charged species across the interface.[43]
Single-cell approach
There are attempts to provide partition coefficients for drugs at a single-cell level.[44][45] This strategy requires methods for the determination of concentrations in individual cells, i.e., with Fluorescence correlation spectroscopy or quantitative Image analysis. Partition coefficient at a single-cell level provides information on cellular uptake mechanism.[45]
Prediction
There are many situations where prediction of partition coefficients prior to experimental measurement is useful. For example, tens of thousands of industrially manufactured chemicals are in common use, but only a small fraction have undergone rigorous toxicological evaluation. Hence there is a need to prioritize the remainder for testing. QSAR equations, which in turn are based on calculated partition coefficients, can be used to provide toxicity estimates.[46][47] Calculated partition coefficients are also widely used in drug discovery to optimize screening libraries[48][49] and to predict druglikeness of designed drug candidates before they are synthesized.[50] As discussed in more detail below, estimates of partition coefficients can be made using a variety of methods, including fragment-based, atom-based, and knowledge-based that rely solely on knowledge of the structure of the chemical. Other prediction methods rely on other experimental measurements such as solubility. The methods also differ in accuracy and whether they can be applied to all molecules, or only ones similar to molecules already studied.
Atom-based
Standard approaches of this type, using atomic contributions, have been named by those formulating them with a prefix letter: AlogP,[51] XlogP,[52] MlogP,[53] etc. A conventional method for predicting log P through this type of method is to parameterize the distribution coefficient contributions of various atoms to the overall molecular partition coefficient, which produces a parametric model. This parametric model can be estimated using constrained least-squares estimation, using a training set of compounds with experimentally measured partition coefficients.[51][53][54] In order to get reasonable correlations, the most common elements contained in drugs (hydrogen, carbon, oxygen, sulfur, nitrogen, and halogens) are divided into several different atom types depending on the environment of the atom within the molecule. While this method is generally the least accurate, the advantage is that it is the most general, being able to provide at least a rough estimate for a wide variety of molecules.[53]
Fragment-based
The most common of these uses a group contribution method and is termed cLogP. It has been shown that the log P of a compound can be determined by the sum of its non-overlapping molecular fragments (defined as one or more atoms covalently bound to each other within the molecule). Fragmentary log P values have been determined in a statistical method analogous to the atomic methods (least-squares fitting to a training set). In addition, Hammett-type corrections are included to account of electronic and steric effects. This method in general gives better results than atomic-based methods, but cannot be used to predict partition coefficients for molecules containing unusual functional groups for which the method has not yet been parameterized (most likely because of the lack of experimental data for molecules containing such functional groups).[21]:125ff[23]:1–193
Knowledge-based
A typical data-mining-based prediction uses support-vector machines,[55] decision trees, or neural networks.[56] This method is usually very successful for calculating log P values when used with compounds that have similar chemical structures and known log P values. Molecule mining approaches apply a similarity-matrix-based prediction or an automatic fragmentation scheme into molecular substructures. Furthermore, there exist also approaches using maximum common subgraph searches or molecule kernels.
Log D from log P and pKa
For cases where the molecule is un-ionized:[13][14]
- [math]\displaystyle{ \log D \cong \log P. }[/math]
For other cases, estimation of log D at a given pH, from log P and the known mole fraction of the un-ionized form, [math]\displaystyle{ f^0 }[/math], in the case where partition of ionized forms into non-polar phase can be neglected, can be formulated as[13][14]
- [math]\displaystyle{ \log D \cong \log P + \log \left(f^0\right). }[/math]
The following approximate expressions are valid only for monoprotic acids and bases:[13][14]
- [math]\displaystyle{ \begin{align} \log D_\text{acids} &\cong \log P + \log\left[\frac{1}{1 + 10^{\mathrm{p}H - \mathrm{p}K_a}}\right], \\ \log D_\text{bases} &\cong \log P + \log\left[\frac{1}{1 + 10^{\mathrm{p}K_a - \mathrm{pH}}}\right]. \end{align} }[/math]
Further approximations for when the compound is largely ionized:[13][14]
- for acids with [math]\displaystyle{ \mathrm{pH} - \mathrm{p}K_a \gt 1 }[/math], [math]\displaystyle{ \log D_\text{acids} \cong \log P + \mathrm{p}K_a - \mathrm{pH} }[/math],
- for bases with [math]\displaystyle{ \mathrm{p}K_a - \mathrm{pH} \gt 1 }[/math], [math]\displaystyle{ \log D_\text{bases} \cong \log P - \mathrm{p}K_a + \mathrm{pH} }[/math].
For prediction of pKa, which in turn can be used to estimate log D, Hammett type equations have frequently been applied.[57][58]
Log P from log S
If the solubility, S, of an organic compound is known or predicted in both water and 1-octanol, then log P can be estimated as[46][59]
- [math]\displaystyle{ \log P = \log S_\text{o} - \log S_\text{w}. }[/math]
There are a variety of approaches to predict solubilities, and so log S.[60][61]
Octanol-water partition coefficient
The partition coefficient between n-Octanol and water is known as the n-octanol-water partition coefficient , or Kow.[62] It is also frequently referred to by the symbol P, especially in the English literature. It is also known as n-octanol-water partition ratio.[63][64][65]
Kow, being a type of partition coefficient, serves as a measure of the relationship between lipophilicity (fat solubility) and hydrophilicity (water solubility) of a substance. The value is greater than one if a substance is more soluble in fat-like solvents such as n-octanol, and less than one if it is more soluble in water.[citation needed]
Example values
Values for log Kow typically range between -3 (very hydrophilic) and +10 (extremely lipophilic/hydrophobic).[66]
The values listed here[67] are sorted by the partition coefficient. Acetamide is hydrophilic, and 2,2′,4,4′,5-Pentachlorobiphenyl is lipophilic.
| Substance | log KOW | T | Reference |
|---|---|---|---|
| Acetamide | −1.155 | 25 °C | |
| Methanol | −0.824 | 19 °C | |
| Formic acid | −0.413 | 25 °C | |
| Diethyl ether | 0.833 | 20 °C | |
| p-Dichlorobenzene | 3.370 | 25 °C | |
| Hexamethylbenzene | 4.610 | 25 °C | |
| 2,2′,4,4′,5-Pentachlorobiphenyl | 6.410 | Ambient |
See also
- Medicine:Blood–gas partition coefficient – Measure of solubility of general anaesthetics in blood
- Chemistry:Cheminformatics – Interdisciplinary science
- Lipinski's rule of five – Rule of thumb to predict if a chemical compound is likely to be an orally active drug
- Chemistry:Lipophilic efficiency – Parameter used in drug design
- Chemistry:Distribution law – Generalisation describing the distribution of a solute between two non miscible solvents.
- Chemistry:ITIES – Electrochemical interface that is either polarisable or polarised
- Chemistry:Ionic partition diagram
References
- ↑ "4.2.4: Partition and Distribution Coefficients". Handbook of Essential Pharmacokinetics, Pharmacodynamics and Drug Metabolism for Industrial Scientists. New York: Kluwer Academic/Plenum Publishers. 2001. p. 44. ISBN 978-1-4757-8693-4. https://books.google.com/books?id=yt7pBwAAQBAJ&pg=PA44.
- ↑ 2.0 2.1 "Partition coefficients and their uses". Chem. Rev. 71 (6): 525–616. 1971. doi:10.1021/cr60274a001.
- ↑ Octanol–Water Partition Coefficients: Fundamentals and Physical Chemistry. Wiley Series in Solution Chemistry. 2. Chichester: John Wiley & Sons Ltd.. 1997. pp. 178. ISBN 978-0-471-97397-3.
- ↑ "Chapter 10: Physiological Drug Distribution and Protein Binding". Applied Biopharmaceutics & Pharmacokinetics (6th ed.). New York: McGraw-Hill Medical. 2012. p. 211. ISBN 978-0-07-160393-5.
- ↑ "Chapter 15: General Anesthetic Pharmacology". Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy (2nd ed.). Philadelphia, Pa.: Lippincott Williams & Wilkins. 2008. p. 243. ISBN 978-0-7817-8355-2. https://books.google.com/books?id=az8uSDkB0mgC&q=gas+blood+partition+coefficient&pg=PA243.
- ↑ 6.0 6.1 "Chapter 3: Solidification". Modern Physical Metallurgy (8th ed.). Amsterdam: Elsevier/Butterworth-Heinemann. 2014. pp. 93–120, esp. 106ff. ISBN 978-0-08-098204-5.
- ↑ "Chapter 3: Free Energy and Phase Diagrams". An Introduction to Aspects of Thermodynamics and Kinetics Relevant to Materials Science (3rd ed.). Amsterdam: Elsevier. 2007. p. 98. ISBN 978-0-08-054968-2. https://books.google.com/books?id=a9qMi8IMYmQC&pg=PR6. "Solid–solid equilibria equivalent to the solid–liquid case"
- ↑ "Hydrophobicity and Partitioning". Pharmaceutics: The Science of Medicine Design. OUP Oxford. 2013. p. 129. ISBN 978-0-19-965531-1. https://books.google.com/books?id=lZGcAQAAQBAJ&pg=PT144.
- ↑ 9.0 9.1 "Partition Coefficient". Compendium of Chemical Terminology: IUPAC Recommendations. Oxford: Blackwell Science. 1997. doi:10.1351/goldbook. ISBN 978-0-86542-684-9. http://goldbook.iupac.org/P04437.html.
- ↑ 10.0 10.1 10.2 10.3 10.4 "Lipophilicity Profiles: Theory and Measurement". Pharmacokinetic Optimization in Drug Research: Biological, Physicochemical, and Computational Strategies. Weinheim: Wiley-VCH. 2001. pp. 275–304. doi:10.1002/9783906390437.ch17. ISBN 978-3-906390-22-2.
- ↑ 11.0 11.1 11.2 "Chapter 4: The Hydrophobic Properties of Molecules". Quantitative Drug Design: A critical introduction (2nd ed.). Boca Raton: CRC Press/Taylor & Francis. 2010. pp. 66–73. ISBN 978-1-4200-7099-6. https://archive.org/details/quantitativedrug00mart.
- ↑ 12.0 12.1 12.2 "Lipophilicity Profiles of Ampholytes". Chemical Reviews 97 (8): 3385–3400. 1997. doi:10.1021/cr9601019. PMID 11851494.
- ↑ 13.0 13.1 13.2 13.3 13.4 "Use of distribution coefficients in quantitative structure-activity relationships". Journal of Medicinal Chemistry 20 (1): 53–8. January 1977. doi:10.1021/jm00211a010. PMID 13215.
- ↑ 14.0 14.1 14.2 14.3 14.4 "Distribution coefficient, a convenient term for the relation of predictable physico-chemical properties to metabolic processes". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 18 (3): 331–50. March 1988. doi:10.3109/00498258809041669. PMID 3289270.
- ↑ "Octanol–Water Partition Coefficients". ddbst.com. http://www.ddbst.com/ddb-kow.html.
- ↑ "Interaction of the peptide bond with solvent water: a vapor phase analysis". Biochemistry 17 (1): 201–4. January 1978. doi:10.1021/bi00594a030. PMID 618544.
- ↑ 17.0 17.1 "The partition of organic compounds. between higher alcohols and water". Acta Chem. Scand. 5: 774–780. 1951. doi:10.3891/acta.chem.scand.05-0774. http://actachemscand.org/pdf/acta_vol_05_p0774-0780.pdf.
- ↑ "Separation of Formic and Sulfuric Acids by Extraction". Ind. Eng. Chem. 47 (10): 2114–2122. 1955. doi:10.1021/ie50550a029.
- ↑ 19.0 19.1 "Octanol–Water Partition Coefficients and Aqueous Solubilities of Organic Compounds". NBS Techn. Rep. 81 (2406): S1–56. 1981. http://nepis.epa.gov/Exe/ZyNET.exe/9101OLPC.txt?ZyActionD=ZyDocument&Client=EPA&Index=1981%20Thru%201985&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5CZYFILES%5CINDEX%20DATA%5C81THRU85%5CTXT%5C00000025%5C9101OLPC.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=p%7Cf&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=2.
- ↑ "Reversed phase liquid chromatography of PCBs as a basis for calculation of water solubility and Kow for polychlorobiphenyls". Fresenius' Z. Anal. Chem. 331 (3–4): 295–301. 1988. doi:10.1007/BF00481899.
- ↑ 21.0 21.1 "Chapter 5: Calculation of Octanol-Water Partition Coefficients from Fragments, etc.". Substituent Constants for Correlation Analysis in Chemistry and Biology. New York: John Wiley & Sons Ltd.. 1979. ISBN 978-0-471-05062-9.
- ↑ "Octanol–Water Partition Coefficients of Simple Organic Compounds". Journal of Physical and Chemical Reference Data 18 (3): 1111–1227. 1989. doi:10.1063/1.555833. Bibcode: 1989JPCRD..18.1111S. https://www.nist.gov/data/PDFfiles/jpcrd367.pdf.
- ↑ 23.0 23.1 "Octanol log P". Exploring QSAR, Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society. 1995. ISBN 978-0-8412-3060-6. https://archive.org/details/exploringqsar0001unse.
- ↑ 24.0 24.1 "Calculation of molecular lipophilicity: State-of-the-art and comparison of log P methods on more than 96,000 compounds". Journal of Pharmaceutical Sciences 98 (3): 861–93. March 2009. doi:10.1002/jps.21494. PMID 18683876.
- ↑ "Chapter 2.2: Pharmacokinetic Phase: ADME". Retrometabolic Drug Design and Targeting. John Wiley & Sons, Inc.. 2012. ISBN 978-1-118-40776-9. https://books.google.com/books?id=Hjxe9jTGUfQC&q=partition+coefficient+drug+absorption+reach+target&pg=PT38.
- ↑ "The influence of drug-like concepts on decision-making in medicinal chemistry". Nature Reviews. Drug Discovery 6 (11): 881–90. November 2007. doi:10.1038/nrd2445. PMID 17971784.
- ↑ Role of Physicochemical Properties and Ligand Lipophilicity Efficiency in Addressing Drug Safety Risks. Annual Reports in Medicinal Chemistry. 45. 2010. pp. 381–391. doi:10.1016/S0065-7743(10)45023-X. ISBN 978-0-12-380902-5.
- ↑ "Recent advances in the brain targeting of neuropharmaceuticals by chemical delivery systems". Advanced Drug Delivery Reviews 36 (2–3): 229–254. April 1999. doi:10.1016/S0169-409X(98)00090-8. PMID 10837718.
- ↑ "Nonlinear dependence of biological activity on hydrophobic character: the bilinear model". Il Farmaco; Edizione Scientifica 34 (3): 248–76. March 1979. PMID 43264.
- ↑ "Lipophilicity and biological activity. Drug transport and drug distribution in model systems and in biological systems". Arzneimittel-Forschung 29 (8): 1067–80. 1979. PMID 40579.
- ↑ "Solvation energy in protein folding and binding". Nature 319 (6050): 199–203. 1986. doi:10.1038/319199a0. PMID 3945310. Bibcode: 1986Natur.319..199E.
- ↑ "What determines the strength of noncovalent association of ligands to proteins in aqueous solution?". Proceedings of the National Academy of Sciences of the United States of America 90 (18): 8402–6. September 1993. doi:10.1073/pnas.90.18.8402. PMID 8378312. Bibcode: 1993PNAS...90.8402M.
- ↑ Lipophilicity in Drug Action and Toxicology. New York: John Wiley & Sons Ltd.. 1996. pp. 439 pages. ISBN 978-3-527-29383-4.
- ↑ "The Role of Hydrophobicity in Toxicity Prediction". Current Computer-Aided Drug Design 2 (4): 405–413. 2006. doi:10.2174/157340906778992346.
- ↑ "Partition Coefficients (Kd) for the Modelling of Transport Processes of Radionuclides in Groundwater". Current Computer-Aided Drug Design 2 (4): 405–413. 2006. doi:10.2174/157340906778992346. http://juser.fz-juelich.de/record/154001/files/FZJ-2014-03430.pdf.
- ↑ "Partition coefficients (n-octanol—water) for pesticides". Journal of Chromatography A 642 (1–2): 3–14. July 1993. doi:10.1016/0021-9673(93)80072-G.
- ↑ "Transport, Accumulation and Transformation Processes (Ch. 3), Properties of Chemicals and Estimation Methodologies (Ch. 7), and Procedures of Hazard and Risk Assessment (Ch. 8)". Risk Assessment of Chemicals: An Introduction. Dordrecht: Kluwer Acad. Publ.. 2012. pp. 37–102, and 239–338, esp. 39ff, 240ff, 306, and passim. ISBN 978-0-7923-3740-9. https://books.google.com/books?isbn=9401585202.
- ↑ 38.0 38.1 "The Measurement of Partition Coefficients". Quantitative Structure-Activity Relationships 7 (3): 133–144. 1988. doi:10.1002/qsar.19880070304.
- ↑ "Setup and validation of shake-flask procedures for the determination of partition coefficients (log D) from low drug amounts". European Journal of Pharmaceutical Sciences 76: 181–91. August 2015. doi:10.1016/j.ejps.2015.05.008. PMID 25968358.
- ↑ "Application of high-performance liquid chromatography based measurements of lipophilicity to model biological distribution". Journal of Chromatography A 1037 (1–2): 299–310. May 2004. doi:10.1016/j.chroma.2003.10.084. PMID 15214672.
- ↑ "Water-oil partition profiling of ionized drug molecules using cyclic voltammetry and a 96-well microfilter plate system". Pharmaceutical Research 20 (8): 1317–22. August 2003. doi:10.1023/A:1025025804196. PMID 12948031. http://infoscience.epfl.ch/record/269694/files/11095_2004_Article_468694.pdf.
- ↑ "A new access to Gibbs energies of transfer of ions across liquid|liquid interfaces and a new method to study electrochemical processes at well-defined three-phase junctions" (in en). Electrochemistry Communications 2 (2): 112–118. February 2000. doi:10.1016/S1388-2481(99)00156-3. https://linkinghub.elsevier.com/retrieve/pii/S1388248199001563.
- ↑ "Mechanistic aspects of the electron and ion transport processes across the electrode". Journal of Electroanalytical Chemistry 372 (1–2): 125–135. 1994. doi:10.1016/0022-0728(93)03257-P.
- ↑ Karpińska, Aneta; Pilz, Marta; Buczkowska, Joanna; Żuk, Paweł J.; Kucharska, Karolina; Magiera, Gaweł; Kwapiszewska, Karina; Hołyst, Robert (2021). "Quantitative analysis of biochemical processes in living cells at a single-molecule level: a case of olaparib–PARP1 (DNA repair protein) interactions". Analyst 146 (23): 7131–7143. doi:10.1039/D1AN01769A. PMID 34726203. https://eprints.lancs.ac.uk/id/eprint/161906/1/AN_ART_09_2021_001769.R1_Proof_hi.pdf.
- ↑ 45.0 45.1 Karpinska, Aneta; Magiera, Gaweł; Kwapiszewska, Karina; Hołyst, Robert (2023). "Cellular Uptake of Bevacizumab in Cervical and Breast Cancer Cells Revealed by Single-Molecule Spectroscopy". J. Phys. Chem. Lett. 14 (5): 1272–1278. doi:10.1021/acs.jpclett.2c03590. PMID 36719904.
- ↑ 46.0 46.1 "A General Guidebook for the Theoretical Prediction of Physicochemical Properties of Chemicals for Regulatory Purposes". Chemical Reviews 115 (24): 13093–164. December 2015. doi:10.1021/acs.chemrev.5b00215. PMID 26624238.
- ↑ "The toxicity data landscape for environmental chemicals". Environmental Health Perspectives 117 (5): 685–95. May 2009. doi:10.1289/ehp.0800168. PMID 19479008.
- ↑ "Computational approaches towards the rational design of drug-like compound libraries". Combinatorial Chemistry & High Throughput Screening 4 (6): 453–75. September 2001. doi:10.2174/1386207013330896. PMID 11562252.
- ↑ "Library design for fragment based screening". Current Topics in Medicinal Chemistry 5 (8): 751–62. 2005. doi:10.2174/1568026054637700. PMID 16101415.
- ↑ "Lipophilicity--methods of determination and its role in medicinal chemistry". Acta Poloniae Pharmaceutica 70 (1): 3–18. 2013. PMID 23610954. http://www.ptfarm.pl/pub/File/Acta_Poloniae/2013/1/003.pdf.
- ↑ 51.0 51.1 "Atomic Physicochemical Parameters for Three-Dimensional Structure-Directed Quantitative Structure–Activity Relationships I. Partition Coefficients as a Measure of Hydrophobicity". Journal of Computational Chemistry 7 (4): 565–577. 1986. doi:10.1002/jcc.540070419. https://deepblue.lib.umich.edu/bitstream/2027.42/38274/1/540070419_ftp.pdf.
- ↑ "Computation of octanol-water partition coefficients by guiding an additive model with knowledge". Journal of Chemical Information and Modeling 47 (6): 2140–8. 2007. doi:10.1021/ci700257y. PMID 17985865. http://www.sioc-ccbg.ac.cn/?p=42&software=xlogp3.
- ↑ 53.0 53.1 53.2 "Simple method of calculating octanol/water partition coefficient". Chem. Pharm. Bull. 40 (1): 127–130. 1992. doi:10.1248/cpb.40.127.
- ↑ "Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragmental Methods: An Analysis of AlogP and ClogP Methods". Journal of Physical Chemistry A 102 (21): 3762–3772. 1998. doi:10.1021/jp980230o. Bibcode: 1998JPCA..102.3762G.
- ↑ "SVM approach for predicting LogP". Molecular Diversity 10 (3): 301–9. August 2006. doi:10.1007/s11030-006-9036-2. PMID 17031534.
- ↑ "A neural network based prediction of octanol-water partition coefficients using atomic5 fragmental descriptors". Bioorganic & Medicinal Chemistry Letters 14 (4): 851–3. February 2004. doi:10.1016/j.bmcl.2003.12.024. PMID 15012980.
- ↑ "Chapter 3: Methods of pKa Prediction". pKa Prediction for Organic Acids and Bases. London: Chapman & Hall. 1981. pp. 21–26. doi:10.1007/978-94-009-5883-8. ISBN 978-0-412-22190-3.
- ↑ "Reference Module in Chemistry, Molecular Sciences and Chemical Engineering". Reference Module in Chemistry, Molecular Sciences and Chemical Engineering [Online]. 5. Amsterdam, the Netherlands: Elsevier. 2013. doi:10.1016/B978-0-12-409547-2.02610-X. ISBN 9780124095472.
- ↑ "Correlation of Octanol/Water Solubility Ratios and Partition Coefficients". Journal of Chemical & Engineering Data 40 (3): 623–626. May 1995. doi:10.1021/je00019a019.
- ↑ "Recent advances on aqueous solubility prediction". Combinatorial Chemistry & High Throughput Screening 14 (5): 328–38. June 2011. doi:10.2174/138620711795508331. PMID 21470182.
- ↑ "A review of methods for the calculation of solution free energies and the modelling of systems in solution". Physical Chemistry Chemical Physics 17 (9): 6174–91. March 2015. doi:10.1039/c5cp00288e. PMID 25660403. Bibcode: 2015PCCP...17.6174S. https://research-repository.st-andrews.ac.uk/bitstream/10023/6096/1/c5cp00288e.pdf.
- ↑ Octanol-water partition coefficients : fundamentals and physical chemistry. Chichester: Wiley. 1997. ISBN 0-471-97397-1. OCLC 36430034.
- ↑ Multimedia environmental models : the fugacity approach. J. Mark Parnis (Third ed.). Boca Raton, FL. 2021. ISBN 978-1-000-09499-2. OCLC 1182869019. https://www.worldcat.org/oclc/1182869019.
- ↑ "A comparison of log Kow (n-octanol–water partition coefficient) values for non-ionic, anionic, cationic and amphoteric surfactants determined using predictions and experimental methods". Environmental Sciences Europe 31 (1). 2019. doi:10.1186/s12302-018-0176-7.
- ↑ "The power of size. 1. Rate constants and equilibrium ratios for accumulation of organic substances related to octanol-water partition ratio and species weight". Environmental Toxicology and Chemistry 20 (7): 1399–420. July 2001. doi:10.1002/etc.5620200703. PMID 11434281.
- ↑ "Octanol-Water Partition Coefficient Measurement by a Simple 1H NMR Method". ACS Omega 2 (9): 6244–6249. September 2017. doi:10.1021/acsomega.7b01102. PMID 31457869.
- ↑ "Dortmund Data Bank (DDB)". Dortmund Data Bank Software & Separation Technology (DDBST) GmbH. http://www.ddbst.com/.
Further reading
- "Determination of liquid-liquid partition coefficients by separation methods". Journal of Chromatography A 1037 (1–2): 3–14. May 2004. doi:10.1016/j.chroma.2004.01.001. PMID 15214657. https://www.researchgate.net/publication/8492902.
- "Lipophilicity Profiles: Theory and Measurement". Pharmacokinetic Optimization in Drug Research: Biological, Physicochemical, and Computational Strategies. Weinheim: Wiley-VCH. 2001. pp. 275–304. doi:10.1002/9783906390437.ch17. ISBN 978-3-906390-22-2. https://archive.org/details/pharmokineticopt00test.
- Substituent Constants for Correlation Analysis in Chemistry and Biology. New York: John Wiley & Sons Ltd.. 1979. ISBN 978-0-471-05062-9.
- "Getting physical in drug discovery: a contemporary perspective on solubility and hydrophobicity". Drug Discovery Today 15 (15–16): 648–55. August 2010. doi:10.1016/j.drudis.2010.05.016. PMID 20570751.
- "LogD: lipophilicity for ionisable compounds". Chemosphere 72 (10): 1401–8. August 2008. doi:10.1016/j.chemosphere.2008.04.074. PMID 18565570. Bibcode: 2008Chmsp..72.1401K.
- "Recent methodologies for the estimation of n-octanol/water partition coefficients and their use in the prediction of membrane transport properties of drugs". Mini Reviews in Medicinal Chemistry 5 (2): 127–33. February 2005. doi:10.2174/1389557053402765. PMID 15720283.
- "Partition coefficients and their uses". Chem Rev 71 (6): 525–616. 1971. doi:10.1021/cr60274a001.
- Exploring QSAR, Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society. 1995. ISBN 978-0-8412-3060-6. https://archive.org/details/exploringqsar0001unse.
- "Calculation of molecular lipophilicity: State-of-the-art and comparison of log P methods on more than 96,000 compounds". Journal of Pharmaceutical Sciences 98 (3): 861–93. March 2009. doi:10.1002/jps.21494. PMID 18683876.
- "Chapter 4: The Hydrophobic Properties of Molecules". Quantitative Drug Design: A critical introduction (2nd ed.). Boca Raton: CRC Press/Taylor & Francis. 2010. pp. 66–73. ISBN 978-1-4200-7099-6. https://archive.org/details/quantitativedrug00mart.
- "Chapter 3: Solubility and Lipophilicity". Introduction to the Pharmaceutical Sciences (1st ed.). Baltimore, MD: Lippincott Williams & Wilkins. 2007. pp. 34–37. ISBN 978-0-7817-4478-2. https://books.google.com/books?id=E-Pj1q1PX9cC&q=Partition+coefficient&pg=PA36.
- Partition Coefficient: Determination and Estimation (1st ed.). New York: Pergamon Press. 1986. ISBN 978-0-08-033649-7.
- Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry. Wiley Series in Solution Chemistry. 2. Chichester: John Wiley & Sons Ltd.. 1997. ISBN 978-0-471-97397-3.
External links
- vcclab.org. Overview of the many logP and other physical property calculators available commercially and on-line.
- XLOGP3, a simple LogP estimater used by PubChem (release 2021.05.07)
 |

