Chemistry:Plutonium(IV) nitrate
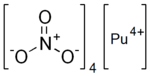
| |
| Names | |
|---|---|
| Other names
Plutonium tetranitrate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Molar mass | 492.02 |
| Appearance | Dark green crystals (hydrates) |
| Soluble | |
| Hazards | |
| Main hazards | Extremely toxic(T+) and radioactive; carcinogen |
| GHS pictograms |    
|
| GHS Signal word | Warning |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Neptunium(IV) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Plutonium (IV) nitrate is an inorganic compound, a salt of plutonium and nitric acid with the chemical formula Pu(NO3)4. The compound dissolves in water and forms crystalline hydrates as dark green crystals.[1][2]
Synthesis
Crystals of dark green to black-green composition Pu(NO3)4•5H2O precipitate with a slow (months) evaporation of a solution of a plutonium (IV) compound in nitric acid.[3][4]
Physical properties
Plutonium (IV) nitrate forms a crystalline hydrate of the composition Pu(NO3)4•5H2O—dark green crystals of rhombic crystal structure, space group F dd2, cell parameters: a = 1.114 nm, b = 2.258 nm, c = 1.051 nm, Z = 8.
Crystalline hydrate melts in its own crystallization water at 95–100 °C.
It dissolves well in nitric acid (dark green solution) and water (brown solution). Also dissolves in acetone and ether.
Chemical properties
When heated to 150–180 °C, it decomposes with autooxidation to plutonium (VI) with the formation of plutonyl nitrate (PuO2(NO3)2). Upon evaporation of concentrated nitric acid solutions of plutonium nitrate and alkali metal nitrates, double nitrates of the composition Me2[Pu(NO3)6] are formed, where Me = Cs+, Rb+, K+, Tl+, NH4+, analogous to ceric ammonium nitrate.
Toxicity
Plutonium nitrate is both radioactive and extremely toxic due to its high solubility in water.
References
- ↑ Allen, P. G.; Veirs, D. K.; Conradson, S. D.; Smith, C. A.; Marsh, S. F. (January 1996). "Characterization of Aqueous Plutonium(IV) Nitrate Complexes by Extended X-ray Absorption Fine Structure Spectroscopy". Inorganic Chemistry 35 (10): 2841–2845. doi:10.1021/ic9511231. https://pubs.acs.org/doi/10.1021/ic9511231. Retrieved 16 August 2021.
- ↑ Kubic, William; Jackson, J. (9 March 2012). "A thermodynamic model of plutonium (IV) nitrate solutions". Journal of Radioanalytical and Nuclear Chemistry 293 (2): 601–612. doi:10.1007/s10967-012-1703-4. ISSN 1588-2780. https://akjournals.com/view/journals/10967/293/2/article-p601.xml. Retrieved 16 August 2021.
- ↑ Baroncelli, F.; Scibona, G.; Zifferero, M. (1 November 1962). "The extraction of Pu(IV) nitrate by long chain tertiary amines nitrates" (in en). Journal of Inorganic and Nuclear Chemistry 24 (5): 541–546. doi:10.1016/0022-1902(62)80241-3. ISSN 0022-1902. https://www.sciencedirect.com/science/article/abs/pii/0022190262802413. Retrieved 16 August 2021.
- ↑ Nakahara, Masaumi; Kaji, Naoya; Yano, Kimihiko; Shibata, Atsuhiro; Takeuchi, Masayuki; Okano, Masanori; Kuno, Takehiko (2013). "Nitric Acid Concentration Dependence of Dicesium Plutonium(IV) Nitrate Formation during Solution Growth of Uranyl Nitrate Hexahydrate". Journal of Chemical Engineering of Japan 46: 56–62. doi:10.1252/jcej.12we175. https://www.jstage.jst.go.jp/article/jcej/advpub/0/advpub_12we175/_pdf. Retrieved 16 August 2021.
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)−4 | C | NO−3, NH4NO3 |
O | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd(NO3)3 | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |


