Chemistry:Plutonium(III) fluoride
| |
| Names | |
|---|---|
| IUPAC name
Plutonium(III) fluoride
| |
| Systematic IUPAC name
Plutonium(3+) fluoride | |
| Other names
Plutonic fluoride
Plutonium fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| F3Pu | |
| Molar mass | 301 g·mol−1 |
| Appearance | Violet, opaque crystals |
| Density | 9.3 g cm−3 |
| Melting point | 1,396 °C (2,545 °F; 1,669 K)[2] |
| Boiling point | 2,000 °C (3,630 °F; 2,270 K) (decomposes)[1] |
| Related compounds | |
Other anions
|
Plutonium(III) chloride |
Other cations
|
Samarium(III) fluoride |
Related fluoroplutoniums
|
Plutonium tetrafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
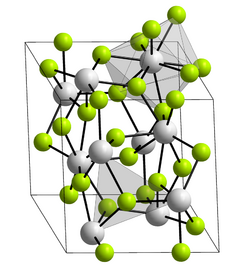
Plutonium(III) fluoride or plutonium trifluoride is the chemical compound composed of plutonium and fluorine with the formula PuF3. This salt forms violet crystals. Plutonium(III) fluoride has the LaF3 structure where the coordination around the plutonium atoms is complex and usually described as tri-capped trigonal prismatic.[3]
Reactions
A plutonium(III) fluoride precipitation method has been investigated as an alternative to the typical plutonium peroxide method of recovering plutonium from solution, such as that from a nuclear reprocessing plant.[4] A 1957 study by the Los Alamos National Laboratory reported a less effective recovery than the traditional method,[5] while a more recent study sponsored by the United States Office of Scientific and Technical Information found it to be one of the more effective methods.[6]
Plutonium(III) fluoride can be used for manufacture of the plutonium-gallium alloy instead of more difficult to handle metallic plutonium.
References
- ↑ Chemistry: Periodic Table: Plutonium: compound data (plutonium (III) fluoride), WebElements, http://202.114.88.54/g/web18/wangluo/webelements/webelements/compounds/text/pu/f3pu1-13842836.html, retrieved 2008-06-20[yes|permanent dead link|dead link}}]
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 113, ISBN 0-8493-0594-2, https://books.google.com/books?id=lFjg0L-uOxoC&q=%22Plutonium(III)+fluoride+%22&pg=PT1110, retrieved 2008-06-20
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN:0-19-855370-6.
- ↑ Gupta, C. K.; Mukherjee, T. K. (1990), Hydrometallurgy in Extraction Processes, 2, CRC Press, pp. 206–208, ISBN 0-8493-6805-7, OCLC 21197603, https://books.google.com/books?id=IV4iOAESyTMC&q=%22plutonium+peroxide%22&pg=PA206, retrieved 2008-06-20
- ↑ Winchester, R. S. (1957), Aqueous Decontamination of Plutonium from Fission Product Elements, Los Alamos, NM: Los Alamos Scientific Laboratory of the University of California (published 1958), pp. 9–10, http://www.fas.org/sgp/othergov/doe/lanl/lib-www/la-pubs/00191208.pdf, retrieved 2008-06-20
- ↑ Martella, L. L.; Saba, M. T.; Campbell, G. K. (1984), Laboratory-scale evaluations of alternative plutonium precipitation methods, United States Office of Scientific and Technical Information, doi:10.2172/5318991, https://digital.library.unt.edu/ark:/67531/metadc1067306/
 |

