Chemistry:Plutonium tetrafluoride
 | |
| |
| Names | |
|---|---|
| IUPAC name
Plutonium(IV) fluoride
| |
| Other names
Plutonium tetrafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| PuF4 | |
| Molar mass | 320 g/mol |
| Appearance | reddish-brown monoclinic crystals |
| Density | 7.1 g/cm3 |
| Melting point | 1,027 °C (1,881 °F; 1,300 K) |
| Structure | |
| Monoclinic, mS60 | |
| C12/c1, No. 15 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Plutonium(IV) fluoride is a chemical compound with the formula (PuF4). This salt is generally a brown solid but can appear a variety of colors depending on the grain size, purity, moisture content, lighting, and presence of contaminants.[4][5] Its primary use in the United States has been as an intermediary product in the production of plutonium metal for nuclear weapons usage.[3]
Formation
Plutonium(IV) fluoride is produced in the reaction between plutonium dioxide (PuO2) or plutonium(III) fluoride (PuF3) with hydrofluoric acid (HF) in a stream of oxygen (O2) at 450 to 600 °C. The main purpose of the oxygen stream is to avoid reduction of the product by hydrogen gas, small amounts of which are often found in HF.[6]
- PuO2 + O2 + 4 HF → PuF4 + O2 + 2 H2O
- 4 PuF3 + O2 + 4 HF → 4 PuF4 + 2 H2O
Laser irradiation of plutonium hexafluoride (PuF6) at wavelengths under 520 nm causes it to decompose into plutonium pentafluoride (PuF5) and fluorine; if this is continued, plutonium(IV) fluoride is obtained.[7]
Properties
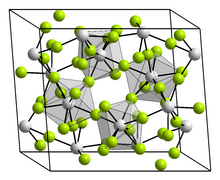
In terms of its structure, solid plutonium(IV) fluoride features 8-coordinate Pu centers interconnected by doubly bridging fluoride ligands.[8]
Reaction of plutonium tetrafluoride with barium, calcium, or lithium at 1200 °C give Pu metal:[4][5][3]
- PuF4 + 2 Ba → 2 BaF2 + Pu
- PuF4 + 2 Ca → 2 CaF2 + Pu
- PuF4 + 4 Li → 4 LiF + Pu

References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–76, ISBN 0-8493-0594-2
- ↑ Pfeiffer, Martin (March 3, 2019). "FOI 2019-00371.Loaded powder pan at RMC line". https://osf.io/5md4c/.
- ↑ 3.0 3.1 3.2 United States Department of Energy (1997). Linking Legacies: Connecting the Cold War Nuclear Weapons Production Processes to Their Environmental Consequences. Washington D.C.: United States Department of Energy. pp. 184; passim. https://www.energy.gov/sites/prod/files/2014/03/f8/Linking_Legacies.pdf.
- ↑ 4.0 4.1 Baldwin, Charles E.; Navratil, James D. (1983-05-19). "Plutonium Process Chemistry at Rocky Flats". in Carnall, William T.; Choppin, Gregory R. (in en). Plutonium Chemistry. ACS Symposium Series. 216. AMERICAN CHEMICAL SOCIETY. pp. 369–380. doi:10.1021/bk-1983-0216.ch024. ISBN 9780841207721.
- ↑ 5.0 5.1 Christensen, Eldon L.; Grey, Leonard W.; Navratil, James D.; Schulz, Wallace W. (1983-05-19). "Present Status and Future Directions of Plutonium Process Chemistry". in Carnall, William T.; Choppin, Gregory R. (in en). Plutonium Chemistry. ACS Symposium Series. 216. AMERICAN CHEMICAL SOCIETY. pp. 349–368. doi:10.1021/bk-1983-0216.ch023. ISBN 9780841207721.
- ↑ Gmelins Handbuch der anorganischen Chemie, System Nr. 71, Transurane, Teil C, pp. 104–107.
- ↑ , Sherman W. & George M. Campbell"Photochemical preparation of plutonium pentafluoride" patent 4670239, issued 1987-06-02
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Pfeiffer, Martin (March 3, 2019). "PuF4 Pics ORO 2019 00475-FN Final Response 20190312_Page_07_Image_0001". https://osf.io/27xzs/.
 |

