Chemistry:Manganese pentacarbonyl bromide
From HandWiki
| |

| |
| Names | |
|---|---|
| Other names
bromopentacarbonylmanganese
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
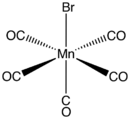
| C5BrMnO5 | |
| Molar mass | 274.892 g·mol−1 |
| Appearance | orange solid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312, H332 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Manganese pentacarbonyl bromide is an organomanganese compound with the formula BrMn(CO)5. It is a bright orange solid that is a precursor to other manganese complexes. The compound is prepared by treatment of dimanganese decacarbonyl with bromine:[1]
- Mn2(CO)10 + Br2 → 2 BrMn(CO)5
The complex undergoes substitution by a variety of donor ligands (L), e.g. to give derivatives of the type BrMn(CO)3L2.
The complex adopts an octahedral coordination geometry.[2]
References
- ↑ King, R. B. (1965). Organometallic Syntheses. Volume 1 Transition-Metal Compounds. New York: Academic Press. ISBN 0-444-42607-8.
- ↑ J. G. Hernandez; I. S. Butler; T. Friščić (214). "Multi-step and multi-component organometallic synthesis in one pot using orthogonal mechanochemical reactions". Chemical Science 5 (9): 3576. doi:10.1039/C4SC01252F.
 |

