Chemistry:Manganese(II,III) oxide
| |
| Names | |
|---|---|
| IUPAC name
manganese(II) dimanganese(III) oxide
| |
| Other names
Manganese tetroxide; Manganese oxide, Manganomanganic oxide, Trimanganese tetraoxide, Trimanganese tetroxide[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| Mn3O4 MnO·Mn2O3 | |
| Molar mass | 228.812 g/mol |
| Appearance | brownish-black powder[1] |
| Density | 4.86 g/cm3 |
| Melting point | 1,567 °C (2,853 °F; 1,840 K) |
| Boiling point | 2,847 °C (5,157 °F; 3,120 K) |
| insoluble | |
| Solubility | soluble in HCl |
| +12,400·10−6 cm3/mol | |
| Structure | |
| Spinel (tetragonal), tI28 | |
| I41/amd, No. 141 | |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
C 5 mg/m3[1] |
REL (Recommended)
|
None established[1] |
IDLH (Immediate danger)
|
N.D.[1] |
| Thermochemistry | |
Std molar
entropy (S |
149 J·mol−1·K−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−1387 kJ·mol−1[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Manganese(II,III) oxide is the chemical compound with formula Mn3O4. Manganese is present in two oxidation states +2 and +3 and the formula is sometimes written as MnO·Mn2O3. Mn3O4 is found in nature as the mineral hausmannite.
Preparation
Mn3O4 formed when any manganese oxide is heated in air above 1000 °C.[3] Considerable research has centred on producing nanocrystalline Mn3O4 and various syntheses that involve oxidation of MnII or reduction of MnVI.[4][5][6]
Reactions
Mn3O4 has been found to act as a catalyst for a range of reactions e.g. the oxidation of methane and carbon monoxide;[7][8] the decomposition of NO,[9] the reduction of nitrobenzene[10] and the catalytic combustion of organic compounds.[11]
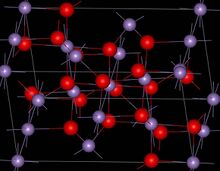
Structure
Mn3O4 has the spinel structure, where the oxide ions are cubic close packed and the MnII occupy tetrahedral sites and the MnIII octahedral sites.[3] The structure is distorted due to the Jahn–Teller effect.[3] At room temperature Mn3O4 is paramagnetic, below 41-43 K, it is ferrimagnetic[12] although this has been reported as reducing in nanocrystalline samples to around 39 K.[13]
Uses
Mn3O4 is sometimes used as a starting material in the production of soft ferrites e.g. manganese zinc ferrite,[14] and lithium manganese oxide, used in lithium batteries.[15]
Manganese tetroxide can also be used as a weighting agent while drilling reservoir sections in oil and gas wells.[citation needed]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 NIOSH Pocket Guide to Chemical Hazards. "#0381". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0381.html.
- ↑ 2.0 2.1 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed.. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ↑ 3.0 3.1 3.2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Hausmannite Mn3O4 nanorods: synthesis, characterization and magnetic properties Jin Du et al. Nanotechnology, (2006),17 4923-4928, doi: 10.1088/0957-4484/17/19/024
- ↑ One-step synthesis of Mn3O4 nanoparticles: Structural and magnetic study Vázquez-Olmos A., Redón R, Rodríguez-Gattorno G., Mata-Zamora M.E., Morales-Leal F, Fernández-Osorio A.L, Saniger J.M. Journal of Colloid and Interface Science, 291, 1, (2005), 175-180 doi:10.1016/j.jcis.2005.05.005
- ↑ Use of Carbonaceous Polysaccharide Microspheres as Templates for Fabricating Metal Oxide Hollow Spheres Xiaoming Sun, Junfeng Liu, Yadong Li, Chemistry - A European Journal,(2005), 12, 7, 2039 – 2047, doi:10.1002/chem.200500660
- ↑ The reduction and oxidation behaviour of manganese oxides Stobhe E.R, de Boer A.D., Geus J.W., Catalysis Today. (1999), 47, 161–167. doi:10.1016/S0920-5861(98)00296-X
- ↑ An in situ XRD investigation of singly and doubly promoted manganese oxide methane coupling catalysts.Moggridge G.D, Rayment T, Lambert R.M. Journal of Catalysis, (1992), 134, 242–252, doi:10.1016/0021-9517(92)90225-7
- ↑ NO Decomposition over Mn2O3 and Mn3O4. Yamashita T, Vannice A., Journal of Catalysis (1996),163, 158–168, doi:10.1006/jcat.1996.0315
- ↑ Selective reduction of nitrobenzene to nitrosobenzene over different kinds of trimanganese tetroxide catalysts.Wang W.M., Yang Y.N., Zhang J.Y., Applied Catalysis A. (1995), 133, 1, 81–93 doi:10.1016/0926-860X(95)00186-7
- ↑ Catalytic combustion of C3 hydrocarbons and oxygenates over Mn3O4. Baldi M, Finocchio E, Milella F, Busca G., Applied Catalysis B. (1998), 16, 1, 43–51, doi:10.1016/S0926-3373(97)00061-1
- ↑ Magnetic Structure of Mn3O4 by Neutron Diffraction Boucher B., Buhl R., Perrin M., J. Appl. Phys. 42, 1615 (1971); doi:10.1063/1.1660364
- ↑ Synthesis of superparamagnetic Mn3O4 nanocrystallites by ultrasonic irradiation I.K. Gopalakrishnan, N. Bagkar, R. Ganguly and S.K. Kulshreshtha Journal of Crystal Growth 280, 3-4, (2005), 436-441, doi:10.1016/j.jcrysgro.2005.03.060
- ↑ Method of making manganese-zinc ferrite U.S Patent number: 4093688 (1978) Arthur Withop, Roger Emil Travagli
- ↑ Process for preparing lithium manganese oxides, U.S Patent number: 6706443,(2004), Horst Krampitz, Gerhard Wohner
 |
