Chemistry:Gonadorelin
 | |
| Clinical data | |
|---|---|
| Trade names | Factrel, HRF, Kryptocur, Lutrelef, Lutrepulse, Relefact, others |
| Other names | Abbott 41070; AY-24031; Hoe-471; RU-19847 |
| Routes of administration | intravenous, subcutaneous[1] |
| Drug class | GnRH analogue; GnRH agonist; Progonadotropin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hydrolysis[1] |
| Elimination half-life | 10–40 minutes[1] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
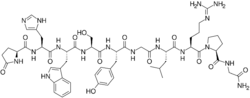
| Formula | C55H75N17O13 |
| Molar mass | 1182.311 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Gonadorelin is a gonadotropin-releasing hormone agonist (GnRH agonist) which is used in fertility medicine and to treat amenorrhea and hypogonadism.[2][3][4][5][6][7] It is also used in veterinary medicine.[3][5] The medication is a form of the endogenous GnRH and is identical to it in chemical structure.[2][3][5][7] It is given by injection into a blood vessel or fat or as a nasal spray.[5][1][7]
Medical uses
Gonadorelin is used as a diagnostic agent to assess pituitary gland function.[1] It is also used in the treatment of primary hypothalamic amenorrhea, hypogonadotropic hypogonadism (e.g., Kallmann syndrome), delayed puberty, cryptorchidism, and infertility.[7][8][1] Unlike other GnRH analogues, it is not used to suppress sex hormone production.[9]
Available forms
Gonadorelin is available in a portable infusion pump that provides pulsatile subcutaneous administration of the drug.[7][8] The usual dosage delivered is 5 to 20 μg of gonadorelin per pulse every 90 to 120 minutes.[7][8] It is also available in solution form for intravenous or subcutaneous injection and as a nasal spray.[7]
Pharmacology
Pharmacodynamics
Gonadorelin is an agonist of the GnRH receptor and is used to induce the secretion of the gonadotropins follicle-stimulating hormone and luteinizing hormone from the pituitary gland and to increase sex hormone production by the gonads.[7][8]
Pharmacokinetics
Gonadorelin has a distribution half-life of 2 to 10 minutes and a very short terminal half-life of 10 to 40 minutes.[1] It is metabolized by hydrolysis into smaller peptide components.[1]
History
Gonadorelin was available for medical use, under the brand name Factrel, as early as 1978.[10]
Society and culture
Generic names
Gonadorelin is the generic name of the drug and its INN, BAN, and JAN, while gonadorelina is its DCIT and gonadoréline is its DCF. The diacetate salt is known as gonadorelin acetate and this is its USAN, BANM, and JAN, while the hydrochloride salt is known as gonadorelin hydrochloride and this is its USAN and BANM.[2][3][5]
Brand names
Free alcohol gonadorelin has been marketed under the brand names Cryptocur, Cystoréline, Fertagyl, GnRH Serono, Gonadorelin, HRF, Kryptocur, LH-RH, Luforan, Pulstim, Relefact, Relisorm L, Stimu-LH, and Wyeth-Ayest HRF.[3][5] Gonadorelin diacetate has been marketed under the brand names Kryptocur, LHRH Ferring, Lutamin, Lutrelef, Lutrepulse, Relisorm L, and Relisorm.[3][5] GnRH hydrochloride has been marketed under the brand names Factrel, HRF, and Luforan.[3][5] Additional brand names of gonadorelin and its salts include Acegon, Conceptyl, Cystorelin, Enagon, Equity Oestrus Control, Fertagyl Cattle, Fertiral, Gonabreed, Gonadorelin Interpharm, Gonasyn, Gonavet Veyx, Hypocrine, Improvest, LH RH Tanabe, LHRH Ferring, LH-RH Ferring, LH-RH Tanabe, Oestracton, OvaCyst, Ovsynch, OVsynch, Ovurelin, Ovarelin, and Relefact LH-RH.[5] The majority of these brand names are for veterinary use.[3][5]
Availability
Gonadorelin is available widely throughout the world for veterinary use, including in the United States , Canada , the United Kingdom , Ireland, elsewhere throughout Europe, South Africa , Australia , New Zealand, Japan , Taiwan, and in many other countries.[3][5][11] However, gonadorelin is not available for clinical use in humans in the United States .[12]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Gonadorelin". https://www.drugbank.ca/drugs/DB00644.
- ↑ 2.0 2.1 2.2 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 745–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA745.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 499–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA499.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 136–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA136.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 "Gonadorelin injectable Uses, Side Effects & Warnings". https://www.drugs.com/international/gonadorelin.html.
- ↑ "[Micropump infusion of gonadorelin in the treatment of hypogonadotropic hypogonadism in patients with pituitary stalk interruption syndrome: cases analysis and literature review]" (in zh). Beijing da Xue Xue Bao. Yi Xue Ban = Journal of Peking University. Health Sciences 46 (4): 642–645. August 2014. PMID 25131486.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 "Hormone Substitution in Male Infertility". The Art & Science of Assisted Reproductive Techniques (ART). JP Medical Ltd. 17 July 2017. pp. 731–. ISBN 978-93-86322-82-1. https://books.google.com/books?id=Gc8nDwAAQBAJ&pg=PA731.
- ↑ 8.0 8.1 8.2 8.3 "Peptide and Protein Drugs". Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. 2002. pp. 128–. ISBN 978-0-683-30737-5. https://books.google.com/books?id=qLJ6Bs1Qml4C&pg=PA128.
- ↑ Goodman & Gilman's the Pharmacological Basis of Therapeutics. McGraw-Hill, Health Professions Division. January 1996. p. 1379. ISBN 978-0-07-026266-9. https://books.google.com/books?id=09h_hZiYXgUC.
- ↑ "LH and FSH response to gonadotropin releasing hormone (GnRH) in normospermic, oligospermic and azoospermic men". Archives of Andrology 1 (2): 147–152. 1978. doi:10.3109/01485017808988331. PMID 367302.
- ↑ "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. 2009. pp. 2106–2108. ISBN 978-0-85369-840-1. https://www.medicinescomplete.com/mc/bnf/64/PHP4615-gonadorelin.htm.
- ↑ "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
 |

