Chemistry:Lithium permanganate
From HandWiki
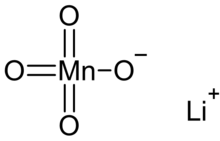
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| LiMnO4 | |
| Molar mass | 125.87 g·mol−1 |
| Appearance | purple[1] |
| solublw | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium permanganate is an inorganic compound with the chemical formula LiMnO4. It can be produced by the reaction of lithium sulfate and barium permanganate, and the trihydrate LiMnO4·3H2O can be crystallized from the solution. It decomposes violently at 199 °C:[2]
- 2 LiMnO
4 → Li
2O + 2MnO
2 + ³/₂ O
2↑
However, later research found that the thermal decomposition products of lithium permanganate are more complex, with products such as Li2MnO3 and LiMn2O4 being generated.[3]
References
- ↑ Pierre Villars; Karin Cenzual; Roman Gladyshevskii (24 July 2017). Handbook. Walter de Gruyter GmbH & Co KG. ISBN 978-3-11-043655-6. https://books.google.com/books?id=ycw0DwAAQBAJ&pg=PT1631.
- ↑ "Zhurnal Neorganicheskoi Khimii (Inorganic Chemistry) is 50". Russian Journal of Inorganic Chemistry 51 (5): 844–845. May 2006. doi:10.1134/s0036023606050287. ISSN 0036-0236. http://dx.doi.org/10.1134/s0036023606050287.
- ↑ Alexander A. Andriiko, Arseniy Ye. Shpak, Yuriy O. Andriyko, José R. García, Sergei A. Khainakov, Nataliya Ye. Vlasenko (May 2012). "Formation of spinel structured compounds in the lithium permanganate thermal decomposition" (in en). Journal of Solid State Electrochemistry 16 (5): 1993–1998. doi:10.1007/s10008-011-1603-5. ISSN 1432-8488. http://link.springer.com/10.1007/s10008-011-1603-5. Retrieved 2020-06-21.
 |

