Chemistry:Lithium citrate
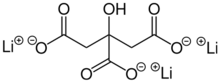
| |
| Names | |
|---|---|
| Other names
Trilithium citrate
trilithium 2-hydroxypropane-1,2,3-tricarboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| Li3C6H5O7 | |
| Molar mass | 209.923 g mol−1 |
| Appearance | Odorless white powder |
| Melting point | decomposes at 105 °C (221 °F; 378 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H319 | |
| P305+351+338 | |
| Flash point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium citrate (Li3C6H5O7) is a lithium salt of citric acid that is used as a mood stabilizer in psychiatric treatment of manic states and bipolar disorder.[1][2][3][4] There is extensive pharmacology of lithium, the active component of this salt.
History
Lithium citrate was one of the lithium salts used to add lithium to drinks and water (lithia water) in the late 19th century and the early 20th century, when there was a general health craze for lithium with it believed to be a cure-all.[5] The soft drink 7Up was at one point named "7Up Lithiated Lemon Soda" when it was formulated in 1929 because it claimed to contain lithium citrate. The beverage was a patent medicine marketed as a cure for hangover. In 1936 the federal government forced the manufacturer to remove a number of health claims, and because "lithium was not an actual ingredient", the name was changed to just "7 Up" in 1937.[6]: §2 Many sources repeat an incorrect version of the story where the name is "Bib-Label Lithiated Lemon-Lime Soda" and the removal happened in 1948 due to a Food and Drug Administration ban.[7][8]
Lithium citrate is used as a mood stabilizer and is used to treat mania, hypomania, depression and bipolar disorder.[9] It can be administered orally in the form of a syrup.[9]
References
- ↑ Medication description
- ↑ "pms-Lithium Citrate - Uses, Side Effects, Interactions - MedBroadcast.com". https://medbroadcast.com/drug/getdrug/pms-lithium-citrate.
- ↑ "Medical use". https://www.nami.org/Template.cfm?Section=About_Medications&Template=/TaggedPage/TaggedPageDisplay.cfm&TPLID=51&ContentID=20820.
- ↑ "Lithium: medicine to control mood disorders such as mania and bipolar disorder". 2020-08-17. https://www.nhs.uk/medicines/lithium/.
- ↑ Friedrich, M. J. (23 June 1999). "Lithium: Proving Its Mettle for 50 Years". JAMA 281 (24): 2271–2273. doi:10.1001/jama.281.24.2271. PMID 10386539.
- ↑ Lockhart, Bill; Brown, Bob (2024). "The Seven-Up Company and 7-Up Bottles The Real Story: A Look at the Myths, the Mystery, and the Magic". Society for Historical Archaeology. https://sha.org/bottle/pdffiles/7-UpStudy1.pdf. Chapter 1 Chapter 2
- ↑ Gielen, Marcel; Edward R. T. Tiekink (2005). Metallotherapeutic drugs and metal-based diagnostic agents: The use of metals in medicine. John Wiley and Sons. p. 3. ISBN 0-470-86403-6. https://archive.org/details/metallotherapeut00giel.
- ↑ "Here's the Gross Thing That Happens when You Mix 7-Up with Lithium". Time, Inc.. 2016-02-20. https://time.com/4231522/7up-lithium-chemical-reaction/.
- ↑ 9.0 9.1 PubChem. "Lithium citrate". https://pubchem.ncbi.nlm.nih.gov/compound/13520.
 |
