Chemistry:Lithium iodate
From HandWiki
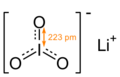
| |
| |
| Names | |
|---|---|
| IUPAC name
Lithium iodate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1479 |
| |
| |
| Properties | |
| LiIO3 | |
| Appearance | White hygroscopic crystals |
| Odor | Odorless |
| Density | 4.487 g/cm3[1] |
| Melting point | 420–450 °C (788–842 °F; 693–723 K)[1][3][5] |
| Anhydrous: 89.4 g/100 mL (10 °C) 82.7 g/100 mL (25 °C) 78.4 g/100 mL (40.1 °C) 73 g/100 mL (75.6 °C)[1] Hemihydrate: 80.2 g/100 mL (18 °C)[2] | |
| Solubility | Insoluble in EtOH[3] |
| −47.0·10−6 cm3/mol | |
| Thermal conductivity | 1.27 W/m·K (a-axis) 0.65 W/m·K (c-axis)[1] |
Refractive index (nD)
|
1.8875 (20 °C) 1.6 (RT) nHe–Ne: 1.8815 (20 °C)[1] 1.5928 (RT)[4] |
| Structure | |
| Hexagonal,[3] hP10[6] | |
| P6322, No. 182[6] | |
| 622[6] | |
a = 5.46(9) Å, c = 5.15(5) Å[6] α = 90°, β = 90°, γ = 120°
| |
| Hazards | |
| GHS pictograms |    [7] [7]
|
| GHS Signal word | Danger |
| H272, H315, H319, H335, H360[7] | |
| P201, P220, P261, P305+351+338, P308+313[7] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Lithium iodate (LiIO3) is a negative uniaxial crystal[1] for nonlinear, acousto-optical and piezoelectric applications. It has been utilized for 347 nm ruby lasers.[9][10]
Properties
Mohs hardness of lithium iodate is 3.5–4. Its linear thermal expansion coefficient at 298 K (25 °C; 77 °F) is 2.8·10−5/°C (a-axis) and 4.8·10−5/°C (c-axis).[1] Its transition to β-form begin at 50 °C (122 °F) and it is irreversible.[5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Rarely Used and Archive Crystals". Nonlinear Optical Crystals: A Complete Survey. 2005. pp. 364–368. doi:10.1007/0-387-27151-1_8. ISBN 978-0-387-27151-4. http://www.webcryst.com/zh/download/finish/6-/477-liio3-lithium-iodate.html. Retrieved 2014-08-08.
- ↑ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York City: D. Van Nostrand Company. p. 374. https://archive.org/details/solubilitiesino01seidgoog.
- ↑ 3.0 3.1 3.2 Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ Polyanskiy, Mikhail. "Refractive index of LiIO3 (Lithium iodate) - Herbst-o". http://refractiveindex.info/?shelf=main&book=LiIO3&page=Herbst-o. Retrieved 2014-08-08.
- ↑ 5.0 5.1 Teyssier, Jeremie; Dantec, Ronan Le; Galez, Christine; Mugnier, Yannick; Bouillot, Jacques; Plenet, Jean-Claude (2003-11-20). Andrews, David L; Gaburro, Zeno; Cartwright, Alexander N et al.. eds. "LiIO3 nanocrystals in SiO2 xerogels, a new material for non-linear optics". Proceedings of SPIE. Nanocrystals, and Organic and Hybrid Nanomaterials 5222 (26): 26. doi:10.1117/12.507309. Bibcode: 2003SPIE.5222...26T. https://www.researchgate.net/publication/252139042.
- ↑ 6.0 6.1 6.2 6.3 Zachariasen, W.H.; Olof, F.A. BartaLars (1931-06-15). "Crystal Structure of Lithium Iodate". Physical Review Letters 37 (12): 1626–1630. doi:10.1103/PhysRev.37.1626. Bibcode: 1931PhRv...37.1626Z.
- ↑ 7.0 7.1 7.2 Sigma-Aldrich Co., Lithium iodate. Retrieved on 2014-08-08.
- ↑ "SDS of Lithium iodate anhydrous". Pfaltz & Bauer, Inc.. https://www.pfaltzandbauer.com/MSDS/L04180%20%20SDS%20%2005202013.pdf. Retrieved 2014-08-08.
- ↑ Risk, W. P.; Gosnell, T. R.; Nurmikko, A. V. (9 January 2003). Compact Blue-Green Lasers. Cambridge University Press. p. 123. ISBN 978-0-521-52103-1. https://books.google.com/books?id=rtLwj5H9JacC&pg=PA123. Retrieved 13 December 2012.
- ↑ Nikogosyan, David N. (4 January 2005). Nonlinear Optical Crystals: A Complete Survey. Springer. p. 371. ISBN 978-0-387-22022-2. https://books.google.com/books?id=ZW9Ynx_Z7kkC&pg=PA371. Retrieved 13 December 2012.
 |

