Chemistry:Lithium chlorate
From HandWiki
[[File:Lithium ion 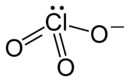 |220px]] |220px]]
| |
| Names | |
|---|---|
| Other names
Chloric acid, lithium salt
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| LiClO3 | |
| Molar mass | 90.39 g/mol |
| Melting point | 127.6 to 129 °C (261.7 to 264.2 °F; 400.8 to 402.1 K)[2][3][4] |
| 241 g/100 mL (0 °C) 459 g/100 mL (25 °C) 777 g/100 mL (60 °C) 2226 g/100 mL (100 °C)[1] | |
| −28.8·10−6 cm3/mol | |
| Related compounds | |
Other anions
|
Lithium chloride Lithium hypochlorite Lithium perchlorate |
Other cations
|
Sodium chlorate Potassium chlorate Caesium chlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium chlorate is the inorganic chemical compound with the formula LiClO3. Like all chlorates, it is an oxidizer and may become unstable and possibly explosive if mixed with organic materials, reactive metal powders, or sulfur.
It can be manufactured by the reaction of hot, concentrated lithium hydroxide with chlorine:
- 3 Cl2 + 6 LiOH → 5 LiCl + LiClO3 + 3 H2O
Lithium chlorate has one of the highest solubilities in water for a chemical compound. It is also a six-electron oxidant. Its electrochemical reduction is facilitated by acid, electrocatalysts and redox mediators. These properties make lithium chlorate a useful oxidant for high energy density flow batteries.[5] Lithium chlorate has a very low melting point for an inorganic ionic salt.
References
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 4–47. ISBN 978-1138561632.
- ↑ Wang, Su-Chee Simon (1983). "The Electrochemistry of Molten Lithium Chlorate and its Possible Use with Lithium in a Battery". Journal of the Electrochemical Society 130 (4): 741–747. doi:10.1149/1.2119796. Bibcode: 1983JElS..130..741W.
- ↑ A. N. Campbell, E. M. Kartzmark, W. B. Maryk (1966). "The Systems Sodium Chlorate - Water - Dioxane and Lithium Chlorate - Water - Dioxane, at 25°". Can. J. Chem. 44 (8): 935–937. doi:10.1139/v66-136.
- ↑ http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=JESOAN000130000004000741000001&idtype=cvips&gifs=yes&ref=no
- ↑ US patent 20140170511
 |
