Chemistry:Oleyl alcohol
| |
| |
| Names | |
|---|---|
| IUPAC name
(Z)-Octadec-9-en-1-ol
| |
| Other names
cis-9-Octadecen-1-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H36O | |
| Molar mass | 268.485 g·mol−1 |
| Density | 0.845-0.855 g/cm3 |
| Melting point | 13 to 19 °C (55 to 66 °F; 286 to 292 K) |
| Boiling point | 330 to 360 °C (626 to 680 °F; 603 to 633 K) |
| Insoluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
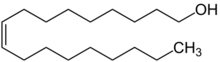
Oleyl alcohol /ˈoʊliˌɪl, ˈoʊliəl/,[1] or cis-9-octadecen-1-ol, is an unsaturated fatty alcohol with the molecular formula C
18H
36O or the condensed structural formula CH
3(CH
2)
7–CH=CH–(CH
2)
8OH. It is a colorless oil, mainly used in cosmetics.[2]
It can be produced by the hydrogenation of oleic acid esters by Bouveault–Blanc reduction, which avoids reduction of the C=C group (as would occur with usual catalytic hydrogenation). The required oleate esters are obtained from beef fat, fish oil, and, in particular, olive oil (from which it gains its name). The original procedure was reported by Louis Bouveault in 1904[3] and subsequently refined.[4][5]
It has uses as a nonionic surfactant, emulsifier, emollient and thickener in skin creams, lotions and many other cosmetic products including shampoos and hair conditioners. It has also been investigated as a carrier for delivering medications through the skin or mucus membranes; particularly the lungs.[6]
See also
- Oleic acid - the corresponding fatty acid
- Oleylamine - the corresponding amine
- Oleamide - the corresponding amide
References
- ↑ "Oleyl" in the McGraw–Hill Dictionary of Scientific & Technical Terms (2003)
- ↑ Noweck, Klaus; Grafahrend, Wolfgang (2006). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_277.pub2.
- ↑ Bouveault, L.; Blanc, G. (1904). "Hydrogénation des éthers des acides possédant en outre les fonctions éther-oxyde ou acétal" (in French). Bull. Soc. Chim. Fr. 31 (3): 1210–1213. http://gallica.bnf.fr/ark:/12148/bpt6k5469971k/f1214.image.
- ↑ Reid, E. E.; Cockerille, F. O.; Meyer, J. D.; Cox, W. M.; Ruhoff, J. R. (1935). "Oleyl Alcohol". Organic Syntheses 15: 51. doi:10.15227/orgsyn.015.0051. http://www.orgsyn.org/demo.aspx?prep=cv2p0468.; Collective Volume, 2, pp. 468
- ↑ Adkins, Homer; Gillespie, R. H. (1935). "Oleyl alcohol". Org. Synth. 29: 51. doi:10.15227/orgsyn.015.0051.
- ↑ Hussain, Alamdar; Arnold, John J.; Khan, Mansoor A.; Ahsan, Fakhrul (2004). "Absorption enhancers in pulmonary protein delivery". J. Control. Release 94 (1): 15–24. doi:10.1016/j.jconrel.2003.10.001. PMID 14684268.
 |


