Earth:Carbon dioxide in Earth's atmosphere

2 concentrations measured at Mauna Loa Observatory from 1958 to 2022 (also called the Keeling Curve). Carbon dioxide concentrations have varied widely over the Earth's 4.54 billion year history. However, in 2013 the daily mean concentration of CO
2 in the atmosphere surpassed 400 parts per million (ppmv)[1] - this level has never been reached since the mid-Pliocene, 2 to 4 million years ago.[2]
In Earth's atmosphere, carbon dioxide is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of several greenhouse gases in the atmosphere of Earth. The current global average concentration of CO2 in the atmosphere is 421 ppm as of May 2022 (0.04%).[3] This is an increase of 50% since the start of the Industrial Revolution, up from 280 ppm during the 10,000 years prior to the mid-18th century.[4][3][5] The increase is due to human activity.[6] Burning fossil fuels is the main cause of these increased CO2 concentrations and also the main cause of climate change.[7] Other large anthropogenic sources include cement production, deforestation, and biomass burning.
While transparent to visible light, carbon dioxide is a greenhouse gas, absorbing and emitting infrared radiation at its two infrared-active vibrational frequencies. CO
2 absorbs and emits infrared radiation at wavelengths of 4.26 μm (2,347 cm−1) (asymmetric stretching vibrational mode) and 14.99 μm (667 cm−1) (bending vibrational mode). It plays a significant role in influencing Earth's surface temperature through the greenhouse effect.[8] Light emission from the Earth's surface is most intense in the infrared region between 200 and 2500 cm−1,[9] as opposed to light emission from the much hotter Sun which is most intense in the visible region. Absorption of infrared light at the vibrational frequencies of atmospheric CO
2 traps energy near the surface, warming the surface and the lower atmosphere. Less energy reaches the upper atmosphere, which is therefore cooler because of this absorption.[10]
Increases in atmospheric concentrations of CO
2 and other long-lived greenhouse gases such as methane, nitrous oxide and ozone increase the absorption and emission of infrared radiation by the atmosphere, causing the observed rise in average global temperature and ocean acidification. Another direct effect is the CO2 fertilization effect. These changes cause a range of indirect effects of climate change on the physical environment, ecosystems and human societies. Carbon dioxide exerts a larger overall warming influence than all of the other greenhouse gases combined.[5] It has an atmospheric lifetime that increases with the cumulative amount of fossil carbon extracted and burned, due to the imbalance that this activity has imposed on Earth's fast carbon cycle.[11] This means that some fraction (a projected 20–35%) of the fossil carbon transferred thus far will persist in the atmosphere as elevated CO
2 levels for many thousands of years after these carbon transfer activities begin to subside.[12][13][14] The carbon cycle is a biogeochemical cycle in which carbon is exchanged between the Earth's oceans, soil, rocks and the biosphere. Plants and other photoautotrophs use solar energy to produce carbohydrate from atmospheric carbon dioxide and water by photosynthesis. Almost all other organisms depend on carbohydrate derived from photosynthesis as their primary source of energy and carbon compounds.
The present atmospheric concentration of CO
2 is the highest for 14 million years.[15] Concentrations of CO
2 in the atmosphere were as high as 4,000 ppm during the Cambrian period about 500 million years ago, and as low as 180 ppm during the Quaternary glaciation of the last two million years.[4] Reconstructed temperature records for the last 420 million years indicate that atmospheric CO
2 concentrations peaked at approximately 2,000 ppm during the Devonian (400 million years) period, and again in the Triassic (220–200 million years) period and were four times current levels during the Jurassic period (201–145 million years).[16][17]
Current concentration and future trends
Current situation
Since the start of the Industrial Revolution, atmospheric CO
2 concentration have been increasing, causing global warming and ocean acidification.[18] In October 2023 the average level of CO
2 in Earth's atmosphere, adjusted for seasonal variation, was 422.17 parts per million by volume (ppm).[19] Figures are published monthly by the National Oceanic & Atmospheric Administration (NOAA).[20][21] The value had been about 280 ppm during the 10,000 years up to the mid-18th century.[4][3][5]
Each part per million of CO
2 in the atmosphere represents approximately 2.13 gigatonnes of carbon, or 7.82 gigatonnes of CO
2.[22]
It was pointed out in 2021 that "the current rates of increase of the concentration of the major greenhouse gases (carbon dioxide, methane and nitrous oxide) are unprecedented over at least the last 800,000 years".[23]:515
It has been estimated that 2,400 gigatons of CO₂ have been emitted by human activity since 1850, with some absorbed by oceans and land, and about 950 gigatons remaining in the atmosphere. Around 2020 the emission rate was over 40 gigatons per year.[24]
Annual and regional fluctuations
Atmospheric CO
2 concentrations fluctuate slightly with the seasons, falling during the Northern Hemisphere spring and summer as plants consume the gas and rising during northern autumn and winter as plants go dormant or die and decay. The level drops by about 6 or 7 ppm (about 50 Gt) from May to September during the Northern Hemisphere's growing season, and then goes up by about 8 or 9 ppm. The Northern Hemisphere dominates the annual cycle of CO
2 concentration because it has much greater land area and plant biomass than the Southern Hemisphere. Concentrations reach a peak in May as the Northern Hemisphere spring greenup begins, and decline to a minimum in October, near the end of the growing season.[25][26]
Concentrations also vary on a regional basis, most strongly near the ground with much smaller variations aloft. In urban areas concentrations are generally higher[27] and indoors they can reach 10 times background levels.
Measurements and predictions made in the recent past
- Estimates in 2001 found that the current carbon dioxide concentration in the atmosphere may be the highest in the last 20 million years.[28] This figure has been corrected down since then, whereby the latest estimate is now 14 million years (estimate from 2013).[15] Most recently, IPCC AR6 (see for example figure 2.34) reports similar levels 3-3.3 mya in the mid-Pliocene warm period. AR6 reports this period as a good proxy for likely climate outcomes with current levels of CO
2. - Data from 2009 found that the global mean CO
2 concentration was rising at a rate of approximately 2 ppm/year and accelerating.[29][30] - The daily average concentration of atmospheric CO
2 at Mauna Loa Observatory first exceeded 400 ppm on 10 May 2013[31][32] although this concentration had already been reached in the Arctic in June 2012.[33] Data from 2013 showed that the concentration of carbon dioxide in the atmosphere is this high "for the first time in 55 years of measurement—and probably more than 3 million years of Earth history."[34] - As of 2018, CO
2 concentrations were measured to be 410 ppm.[29][35]
Measurement techniques
The concentrations of carbon dioxide in the atmosphere are expressed as parts per million by volume (abbreviated as ppmv or just ppm). To convert from the usual ppmv units to ppm mass, multiply by the ratio of the molar weight of CO2 to that of air, i.e. times 1.52 (44.01 divided by 28.96).
The first reproducibly accurate measurements of atmospheric CO2 were from flask sample measurements made by Dave Keeling at Caltech in the 1950s.[36] Measurements at Mauna Loa have been ongoing since 1958. Additionally, measurements are also made at many other sites around the world. Many measurement sites are part of larger global networks. Global network data are often made publicly available.
Data networks
There are several surface measurement (including flasks and continuous in situ) networks including NOAA/ERSL,[37] WDCGG,[38] and RAMCES.[39] The NOAA/ESRL Baseline Observatory Network, and the Scripps Institution of Oceanography Network[40] data are hosted at the CDIAC at ORNL. The World Data Centre for Greenhouse Gases (WDCGG), part of GAW, data are hosted by the JMA. The Reseau Atmospherique de Mesure des Composes an Effet de Serre database (RAMCES) is part of IPSL.
From these measurements, further products are made which integrate data from the various sources. These products also address issues such as data discontinuity and sparseness. GLOBALVIEW-CO
2 is one of these products.[41]
Ongoing ground-based total column measurements began more recently. Column measurements typically refer to an averaged column amount denoted XCO
2, rather than a surface only measurement. These measurements are made by the TCCON. These data are also hosted on the CDIAC, and made publicly available according to the data use policy.[42]
Satellite measurements
Space-based measurements of carbon dioxide are also a recent addition to atmospheric XCO
2 measurements. SCIAMACHY aboard ESA's ENVISAT made global column XCO
2 measurements from 2002 to 2012. AIRS aboard NASA's Aqua satellite makes global XCO
2 measurements and was launched shortly after ENVISAT in 2012. More recent satellites have significantly improved the data density and precision of global measurements. Newer missions have higher spectral and spatial resolutions. JAXA's GOSAT was the first dedicated GHG monitoring satellite to successfully achieve orbit in 2009. NASA's OCO-2 launched in 2014 was the second. Various other satellites missions to measure atmospheric XCO
2 are planned.
Analytical methods to investigate sources of CO2
- The burning of long-buried fossil fuels releases CO
2 containing carbon of different isotopic ratios to those of living plants, enabling distinction between natural and human-caused contributions to CO
2 concentration.[43] - There are higher atmospheric CO
2 concentrations in the Northern Hemisphere, where most of the world's population lives (and emissions originate from), compared to the southern hemisphere. This difference has increased as anthropogenic emissions have increased.[44] - Atmospheric O2 levels are decreasing in Earth's atmosphere as it reacts with the carbon in fossil fuels to form CO
2.[45]
Causes of the current increase
Anthropogenic CO2 emissions
While CO
2 absorption and release is always happening as a result of natural processes, the recent rise in CO
2 levels in the atmosphere is known to be mainly due to human (anthropogenic) activity.[23] Anthropogenic carbon emissions exceed the amount that can be taken up or balanced out by natural sinks.[47] Thus carbon dioxide has gradually accumulated in the atmosphere and, as of May 2022, its concentration is 50% above pre-industrial levels.[3]
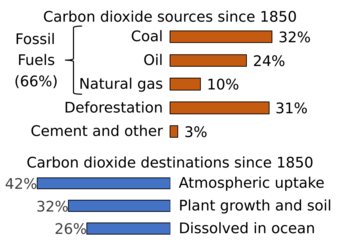
The extraction and burning of fossil fuels, releasing carbon that has been underground for many millions of years, has increased the atmospheric concentration of CO
2.[5][18] As of year 2019 the extraction and burning of geologic fossil carbon by humans releases over 30 gigatonnes of CO
2 (9 billion tonnes carbon) each year.[48] This larger disruption to the natural balance is responsible for recent growth in the atmospheric CO
2 concentration.[35][49] Currently about half of the carbon dioxide released from the burning of fossil fuels is not absorbed by vegetation and the oceans and remains in the atmosphere.[50]
Burning fossil fuels such as coal, petroleum, and natural gas is the leading cause of increased anthropogenic CO
2; deforestation is the second major cause. In 2010, 9.14 gigatonnes of carbon (GtC, equivalent to 33.5 gigatonnes of CO
2 or about 4.3 ppm in Earth's atmosphere) were released from fossil fuels and cement production worldwide, compared to 6.15 GtC in 1990.[51] In addition, land use change contributed 0.87 GtC in 2010, compared to 1.45 GtC in 1990.[51] In the period 1751 to 1900, about 12 GtC were released as CO
2 to the atmosphere from burning of fossil fuels, whereas from 1901 to 2013 the figure was about 380 GtC.[52]
The International Energy Agency estimates that the top 1% of emitters globally each had carbon footprints of over 50 tonnes of CO
2 in 2021, more than 1,000 times greater than those of the bottom 1% of emitters. The global average energy-related carbon footprint is around 4.7 tonnes of CO
2 per person.[53]
Roles in natural processes on Earth
Greenhouse effect
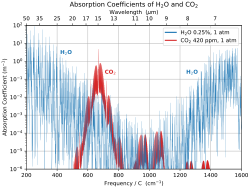
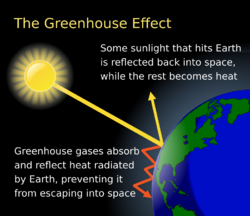
Earth's natural greenhouse effect makes life as we know it possible and carbon dioxide plays a significant role in providing for the relatively high temperature on Earth. The greenhouse effect is a process by which thermal radiation from a planetary atmosphere warms the planet's surface beyond the temperature it would have in the absence of its atmosphere.[54][55][56] Without the greenhouse effect, the Earth's average surface temperature would be about −18 °C (−0.4 °F)[57][58] compared to Earth's actual average surface temperature of approximately 14 °C (57.2 °F).[59]
Water is responsible for most (about 36–70%) of the total greenhouse effect, and the role of water vapor as a greenhouse gas depends on temperature. On Earth, carbon dioxide is the most relevant, direct anthropologically influenced greenhouse gas. Carbon dioxide is often mentioned in the context of its increased influence as a greenhouse gas since the pre-industrial (1750) era. In 2013, the increase in CO2 was estimated to be responsible for 1.82 W m−2 of the 2.63 W m−2 change in radiative forcing on Earth (about 70%).[60]
The concept of atmospheric CO2 increasing ground temperature was first published by Svante Arrhenius in 1896.[61] The increased radiative forcing due to increased CO2 in the Earth's atmosphere is based on the physical properties of CO2 and the non-saturated absorption windows where CO2 absorbs outgoing long-wave energy. The increased forcing drives further changes in Earth's energy balance and, over the longer term, in Earth's climate.[23]
Carbon cycle
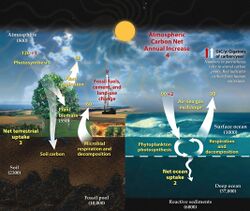
Atmospheric carbon dioxide plays an integral role in the Earth's carbon cycle whereby CO
2 is removed from the atmosphere by some natural processes such as photosynthesis and deposition of carbonates, to form limestones for example, and added back to the atmosphere by other natural processes such as respiration and the acid dissolution of carbonate deposits. There are two broad carbon cycles on Earth: the fast carbon cycle and the slow carbon cycle. The fast carbon cycle refers to movements of carbon between the environment and living things in the biosphere whereas the slow carbon cycle involves the movement of carbon between the atmosphere, oceans, soil, rocks, and volcanism. Both cycles are intrinsically interconnected and atmospheric CO
2 facilitates the linkage.
Natural sources of atmospheric CO
2 include volcanic outgassing, the combustion of organic matter, wildfires and the respiration processes of living aerobic organisms. Man-made sources of CO
2 include the burning of fossil fuels for heating, power generation and transport, as well as some industrial processes such as cement making. It is also produced by various microorganisms from fermentation and cellular respiration. Plants, algae and cyanobacteria convert carbon dioxide to carbohydrates by a process called photosynthesis. They gain the energy needed for this reaction from absorption of sunlight by chlorophyll and other pigments. Oxygen, produced as a by-product of photosynthesis, is released into the atmosphere and subsequently used for respiration by heterotrophic organisms and other plants, forming a cycle with carbon.

2 flows from anthropogenic sources (left) into Earth's atmosphere, land, and ocean sinks (right) since year 1960. Units in equivalent gigatonnes carbon per year.[48]
Most sources of CO
2 emissions are natural, and are balanced to various degrees by similar CO
2 sinks. For example, the decay of organic material in forests, grasslands, and other land vegetation - including forest fires - results in the release of about 436 gigatonnes of CO
2 (containing 119 gigatonnes carbon) every year, while CO
2 uptake by new growth on land counteracts these releases, absorbing 451 Gt (123 Gt C).[63] Although much CO
2 in the early atmosphere of the young Earth was produced by volcanic activity, modern volcanic activity releases only 130 to 230 megatonnes of CO
2 each year.[64] Natural sources are more or less balanced by natural sinks, in the form of chemical and biological processes which remove CO
2 from the atmosphere.
Overall, there is a large natural flux of atmospheric CO
2 into and out of the biosphere, both on land and in the oceans.[65] In the pre-industrial era, each of these fluxes were in balance to such a degree that little net CO
2 flowed between the land and ocean reservoirs of carbon, and little change resulted in the atmospheric concentration. From the human pre-industrial era to 1940, the terrestrial biosphere represented a net source of atmospheric CO
2 (driven largely by land-use changes), but subsequently switched to a net sink with growing fossil carbon emissions.[66] In 2012, about 57% of human-emitted CO
2, mostly from the burning of fossil carbon, was taken up by land and ocean sinks.[67][66]
The ratio of the increase in atmospheric CO
2 to emitted CO
2 is known as the airborne fraction. This ratio varies in the short-term and is typically about 45% over longer (5-year) periods.[66] Estimated carbon in global terrestrial vegetation increased from approximately 740 gigatonnes in 1910 to 780 gigatonnes in 1990.[68]
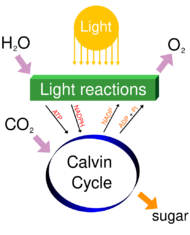
Photosynthesis
Carbon dioxide in the Earth's atmosphere is essential to life and to most of the planetary biosphere. The average rate of energy capture by photosynthesis globally is approximately 130 terawatts,[69][70][71] which is about six times larger than the current power consumption of human civilization.[72] Photosynthetic organisms also convert around 100–115 billion metric tonnes of carbon into biomass per year.[73][74]
Photosynthetic organisms are photoautotrophs, which means that they are able to synthesize food directly from CO
2 and water using energy from light. However, not all organisms that use light as a source of energy carry out photosynthesis, since photoheterotrophs use organic compounds, rather than CO
2, as a source of carbon.[75] In plants, algae and cyanobacteria, photosynthesis releases oxygen. This is called oxygenic photosynthesis. Although there are some differences between oxygenic photosynthesis in plants, algae, and cyanobacteria, the overall process is quite similar in these organisms. Some types of bacteria, however, carry out anoxygenic photosynthesis, which consumes CO
2 but does not release oxygen.[citation needed]
Carbon dioxide is converted into sugars in a process called carbon fixation. Carbon fixation is an endothermic redox reaction, so photosynthesis needs to supply both the source of energy to drive this process and the electrons needed to convert CO
2 into a carbohydrate. This addition of the electrons is a reduction reaction. In general outline and in effect, photosynthesis is the opposite of cellular respiration, in which glucose and other compounds are oxidized to produce CO
2 and water, and to release exothermic chemical energy to drive the organism's metabolism. The two processes take place through a different sequence of chemical reactions, however, and in different cellular compartments.[citation needed]
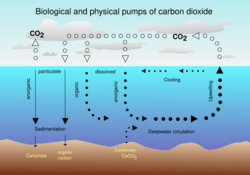
Oceanic carbon cycle
The Earth's oceans contain a large amount of CO
2 in the form of bicarbonate and carbonate ions—much more than the amount in the atmosphere. The bicarbonate is produced in reactions between rock, water, and carbon dioxide. One example is the dissolution of calcium carbonate:
- CaCO3 + CO
2 + H2O ⇌ Ca2+ + 2 HCO−3
Reactions like this tend to buffer changes in atmospheric CO
2. Since the right side of the reaction produces an acidic compound, adding CO
2 on the left side decreases the pH of seawater, a process which has been termed ocean acidification (pH of the ocean becomes more acidic although the pH value remains in the alkaline range). Reactions between CO
2 and non-carbonate rocks also add bicarbonate to the seas. This can later undergo the reverse of the above reaction to form carbonate rocks, releasing half of the bicarbonate as CO
2. Over hundreds of millions of years, this has produced huge quantities of carbonate rocks.
From 1850 until 2022, the ocean has absorbed 26% of total anthropogenic emissions.[18] However, the rate at which the ocean will take it up in the future is less certain. Even if equilibrium is reached, including dissolution of carbonate minerals, the increased concentration of bicarbonate and decreased or unchanged concentration of carbonate ion will give rise to a higher concentration of un-ionized carbonic acid and dissolved CO
2. This higher concentration in the seas, along with higher temperatures, would mean a higher equilibrium concentration of CO
2 in the air.[76][77]
Carbon moves between the atmosphere, vegetation (dead and alive), the soil, the surface layer of the ocean, and the deep ocean.
Effects of current increase
Direct effects
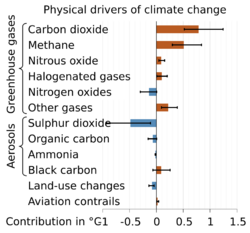
Direct effects of increasing CO2 concentrations in the atmosphere include increasing global temperatures, ocean acidification and a CO2 fertilization effect on plants and crops.[78]
Temperature rise on land
Temperature rise in oceans
Ocean acidification
CO2 fertilization effect
Other direct effects
CO
2 emissions have also led to the stratosphere contracting by 400 meters since 1980, which could affect satellite operations, GPS systems and radio communications.[79]
Indirect effects and impacts
Approaches for reducing CO2 concentrations
File:Following Carbon Dioxide Through the Atmosphere.webm
Carbon dioxide has unique long-term effects on climate change that are nearly "irreversible" for a thousand years after emissions stop (zero further emissions). The greenhouse gases methane and nitrous oxide do not persist over time in the same way as carbon dioxide. Even if human carbon dioxide emissions were to completely cease, atmospheric temperatures are not expected to decrease significantly in the short term. This is because the air temperature is determined by a balance between heating, due to greenhouse gases, and cooling due to heat transfer to the ocean. If emissions were to stop, CO2 levels and the heating effect would slowly decrease, but simultaneously the cooling due to heat transfer would diminish (because sea temperatures would get closer to the air temperature), with the result that the air temperature would decrease only slowly. Sea temperatures would continue to rise, causing thermal expansion and some sea level rise.[76] Lowering global temperatures more rapidly would require carbon sequestration or geoengineering.
Various techniques have been proposed for removing excess carbon dioxide from the atmosphere.
Concentrations in the geologic past

2 over the last 40,000 years, from the Last Glacial Maximum to the present day. The current rate of increase is much higher than at any point during the last deglaciation.
Carbon dioxide is believed to have played an important effect in regulating Earth's temperature throughout its 4.54 billion year history. Early in the Earth's life, scientists have found evidence of liquid water indicating a warm world even though the Sun's output is believed to have only been 70% of what it is today. Higher carbon dioxide concentrations in the early Earth's atmosphere might help explain this faint young sun paradox. When Earth first formed, Earth's atmosphere may have contained more greenhouse gases and CO
2 concentrations may have been higher, with estimated partial pressure as large as 1,000 kPa (10 bar), because there was no bacterial photosynthesis to reduce the gas to carbon compounds and oxygen. Methane, a very active greenhouse gas, may have been more prevalent as well.[80][81]
Carbon dioxide concentrations have shown several cycles of variation from about 180 parts per million during the deep glaciations of the Holocene and Pleistocene to 280 parts per million during the interglacial periods. Carbon dioxide concentrations have varied widely over the Earth's history. It is believed to have been present in Earth's first atmosphere, shortly after Earth's formation. The second atmosphere, consisting largely of nitrogen and CO2 was produced by outgassing from volcanism, supplemented by gases produced during the late heavy bombardment of Earth by huge asteroids.[82] A major part of carbon dioxide emissions were soon dissolved in water and incorporated in carbonate sediments.
The production of free oxygen by cyanobacterial photosynthesis eventually led to the oxygen catastrophe that ended Earth's second atmosphere and brought about the Earth's third atmosphere (the modern atmosphere) 2.4 billion years ago. Carbon dioxide concentrations dropped from 4,000 parts per million during the Cambrian period about 500 million years ago to as low as 180 parts per million 20,000 years ago .[4]
Drivers of ancient-Earth CO2 concentration
On long timescales, atmospheric CO
2 concentration is determined by the balance among geochemical processes including organic carbon burial in sediments, silicate rock weathering, and volcanic degassing. The net effect of slight imbalances in the carbon cycle over tens to hundreds of millions of years has been to reduce atmospheric CO
2. On a timescale of billions of years, such downward trend appears bound to continue indefinitely as occasional massive historical releases of buried carbon due to volcanism will become less frequent (as earth mantle cooling and progressive exhaustion of internal radioactive heat proceed further). The rates of these processes are extremely slow; hence they are of no relevance to the atmospheric CO
2 concentration over the next hundreds or thousands of years.
Photosynthesis in the geologic past
Over the course of Earth's geologic history CO
2 concentrations have played a role in biological evolution. The first photosynthetic organisms probably evolved early in the evolutionary history of life and most likely used reducing agents such as hydrogen or hydrogen sulfide as sources of electrons, rather than water.[83] Cyanobacteria appeared later, and the excess oxygen they produced contributed to the oxygen catastrophe,[84] which rendered the evolution of complex life possible. In recent geologic times, low CO
2 concentrations below 600 parts per million might have been the stimulus that favored the evolution of C4 plants which increased greatly in abundance between 7 and 5 million years ago over plants that use the less efficient C3 metabolic pathway.[85] At current atmospheric pressures photosynthesis shuts down when atmospheric CO
2 concentrations fall below 150 ppm and 200 ppm although some microbes can extract carbon from the air at much lower concentrations.[86][87]
Measuring ancient-Earth CO2 concentration
The most direct method for measuring atmospheric carbon dioxide concentrations for periods before instrumental sampling is to measure bubbles of air (fluid or gas inclusions) trapped in the Antarctic or Greenland ice sheets. The most widely accepted of such studies come from a variety of Antarctic cores and indicate that atmospheric CO
2 concentrations were about 260–280 ppm immediately before industrial emissions began and did not vary much from this level during the preceding 10,000 years.[88][89] The longest ice core record comes from East Antarctica, where ice has been sampled to an age of 800,000 years.[90] During this time, the atmospheric carbon dioxide concentration has varied between 180 and 210 ppm during ice ages, increasing to 280–300 ppm during warmer interglacials.[91][92]
CO
2 mole fractions in the atmosphere have gone up by around 35 percent since the 1900s, rising from 280 parts per million by volume to 387 parts per million in 2009. One study using evidence from stomata of fossilized leaves suggests greater variability, with CO
2 mole fractions above 300 ppm during the period ten to seven thousand years ago,[93] though others have argued that these findings more likely reflect calibration or contamination problems rather than actual CO2 variability.[94][95] Because of the way air is trapped in ice (pores in the ice close off slowly to form bubbles deep within the firn) and the time period represented in each ice sample analyzed, these figures represent averages of atmospheric concentrations of up to a few centuries rather than annual or decadal levels.
Ice cores provide evidence for greenhouse gas concentration variations over the past 800,000 years. Both CO2 and CH4 concentrations vary between glacial and interglacial phases, and these variations correlate strongly with temperature. Direct data does not exist for periods earlier than those represented in the ice core record, a record that indicates that CO2 mole fractions stayed within a range of 180 ppm to 280 ppm throughout the last 800,000 years, until the increase of the last 250 years. However, various proxy measurements and models suggest larger variations in past epochs: 500 million years ago CO2 levels were likely 10 times higher than now.[96]
Various proxy measurements have been used to try to determine atmospheric CO2 concentrations millions of years in the past. These include boron and carbon isotope ratios in certain types of marine sediments, and the numbers of stomata observed on fossil plant leaves.[85]
Phytane is a type of diterpenoid alkane. It is a breakdown product of chlorophyll, and is now used to estimate ancient CO
2 levels.[97] Phytane gives both a continuous record of CO
2 concentrations but it also can overlap a break in the CO
2 record of over 500 million years.[97]
600 to 400 million years ago
There is evidence for high CO
2 concentrations of over 6,000 ppm between 600 and 400 million years ago, and of over 3,000 ppm between 200 and 150 million years ago.[28]
Indeed, higher CO2 concentrations are thought to have prevailed throughout most of the Phanerozoic Eon, with concentrations four to six times current concentrations during the Mesozoic era, and ten to fifteen times current concentrations during the early Palaeozoic era until the middle of the Devonian period, about 400 million years ago.[98][99][100] The spread of land plants is thought to have reduced CO2 concentrations during the late Devonian, and plant activities as both sources and sinks of CO2 have since been important in providing stabilizing feedbacks.[101]
Earlier still, a 200-million year period of intermittent, widespread glaciation extending close to the equator (Snowball Earth) appears to have been ended suddenly, about 550 Ma, by a colossal volcanic outgassing that raised the CO
2 concentration of the atmosphere abruptly to 12%, about 350 times modern levels, causing extreme greenhouse conditions and carbonate deposition as limestone at the rate of about 1 mm per day.[102] This episode marked the close of the Precambrian Eon, and was succeeded by the generally warmer conditions of the Phanerozoic, during which multicellular animal and plant life evolved. No volcanic CO2 emission of comparable scale has occurred since. In the modern era, emissions to the atmosphere from volcanoes are approximately 0.645 billion tons of CO
2 per year, whereas humans contribute 29 billion tons of CO
2 each year.[103][102][104][105]
60 to 5 million years ago
Atmospheric CO
2 concentration continued to fall after about 60 million years ago. About 34 million years ago, the time of the Eocene–Oligocene extinction event and when the Antarctic ice sheet started to take its current form, CO
2 was about 760 ppm,[106] and there is geochemical evidence that concentrations were less than 300 ppm by about 20 million years ago. Decreasing CO
2 concentration, with a tipping point of 600 ppm, was the primary agent forcing Antarctic glaciation.[107] Low CO
2 concentrations may have been the stimulus that favored the evolution of C4 plants, which increased greatly in abundance between 7 and 5 million years ago.[85]
See also
References
- ↑ Showstack, Randy (2013). "Carbon dioxide tops 400 ppm at Mauna Loa, Hawaii". Eos, Transactions American Geophysical Union 94 (21): 192. doi:10.1002/2013eo210004. ISSN 0096-3941. Bibcode: 2013EOSTr..94Q.192S.
- ↑ Montaigne, Fen. "Son of Climate Science Pioneer Ponders A Sobering Milestone". Yale School of Forestry & Environmental Studies. http://e360.yale.edu/feature/keeling_curve_son_of_climate_science_pioneer_on_co2_milestone/2650/.
- ↑ 3.0 3.1 3.2 3.3 "Carbon dioxide now more than 50% higher than pre-industrial levels | National Oceanic and Atmospheric Administration". 3 June 2022. https://www.noaa.gov/news-release/carbon-dioxide-now-more-than-50-higher-than-pre-industrial-levels.
- ↑ 4.0 4.1 4.2 4.3 Eggleton, Tony (2013). A Short Introduction to Climate Change. Cambridge University Press. p. 52. ISBN 9781107618763. https://books.google.com/books?id=jeSwRly2M_cC&q=280&pg=PA52. Retrieved 14 March 2023.
- ↑ 5.0 5.1 5.2 5.3 "The NOAA Annual Greenhouse Gas Index (AGGI) – An Introduction". NOAA Global Monitoring Laboratory/Earth System Research Laboratories. https://www.esrl.noaa.gov/gmd/aggi/.
- ↑ Etheridge, D.M.; L.P. Steele; R.L. Langenfelds; R.J. Francey; J.-M. Barnola; V.I. Morgan (1996). "Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn". Journal of Geophysical Research 101 (D2): 4115–28. doi:10.1029/95JD03410. ISSN 0148-0227. Bibcode: 1996JGR...101.4115E.
- ↑ IPCC (2022) Summary for policy makers in Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change , Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- ↑ Petty, G.W. (2004). "A First Course in Atmospheric Radiation". Eos Transactions 85 (36): 229–51. doi:10.1029/2004EO360007. Bibcode: 2004EOSTr..85..341P.
- ↑ Atkins' Physical Chemistry (8th ed.). W. H. Freeman. 2006. p. 462. ISBN 978-0-7167-8759-4. https://archive.org/details/atkinsphysicalch00pwat/page/462.
- ↑ "Carbon Dioxide Absorbs and Re-emits Infrared Radiation". UCAR Center for Science Education. 2012. https://scied.ucar.edu/carbon-dioxide-absorbs-and-re-emits-infrared-radiation.
- ↑ "How long will global warming last?". RealClimate. 2005-03-15. http://www.realclimate.org/index.php/archives/2005/03/how-long-will-global-warming-last.
- ↑ "Atmospheric lifetime of fossil fuel carbon dioxide". Annual Review of Earth and Planetary Sciences 37 (1): 117–34. 2009. doi:10.1146/annurev.earth.031208.100206. Bibcode: 2009AREPS..37..117A. https://orbi.uliege.be/handle/2268/12933. Retrieved 7 March 2021.
- ↑ "Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis". Atmospheric Chemistry and Physics 13 (5): 2793–2825. 2013. doi:10.5194/acpd-12-19799-2012. https://www.atmos-chem-phys.net/13/2793/2013/. Retrieved 7 March 2021.
- ↑ "Figure 8.SM.4". Intergovernmental Panel on Climate Change Fifth Assessment Report. p. 8SM-16. https://www.ipcc.ch/site/assets/uploads/2018/07/WGI_AR5.Chap_.8_SM.pdf. Retrieved 7 March 2021.
- ↑ 15.0 15.1 Zhang, Yi Ge (28 October 2013). "A 40-million-year history of atmospheric CO
2". Philosophical Transactions of the Royal Society A 371 (2001): 20130096. doi:10.1098/rsta.2013.0096. PMID 24043869. Bibcode: 2013RSPTA.37130096Z. - ↑ "Climate and CO2 in the Atmosphere". http://earthguide.ucsd.edu/virtualmuseum/climatechange2/07_1.shtml.
- ↑ "GEOCARB III: A revised model of atmospheric CO2 over Phanerozoic Time". American Journal of Science 301 (2): 182–204. 2001. doi:10.2475/ajs.301.2.182. Bibcode: 2001AmJS..301..182B. http://www.geocraft.com/WVFossils/Reference_Docs/Geocarb_III-Berner.pdf. Retrieved 15 February 2008.
- ↑ 18.0 18.1 18.2 Friedlingstein, Pierre; O'Sullivan, Michael; Jones, Matthew W.; Andrew, Robbie M.; Gregor, Luke; Hauck, Judith; Le Quéré, Corinne; Luijkx, Ingrid T. et al. (2022-11-11). "Global Carbon Budget 2022". Earth System Science Data 14 (11): 4811–4900. doi:10.5194/essd-14-4811-2022. Bibcode: 2022ESSD...14.4811F.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ↑ "Parts per million" refers to the number of carbon dioxide molecules per million molecules of dry air. "Carbon Dioxide LATEST MEASUREMENT". NASA Global Climate Change. https://climate.nasa.gov/vital-signs/carbon-dioxide. Updated monthly.
- ↑ "Global Monitoring Laboratory - Trends in Atmospheric Carbon Dioxide". National Oceanic & Atmospheric Administration. https://gml.noaa.gov/ccgg/trends/. Latest figure, and graphs of trend; frequently updated
- ↑ "Table of atmospheric CO₂ since 1958, updated monthly". National Oceanic & Atmospheric Administration. https://gml.noaa.gov/webdata/ccgg/trends/co2/co2_mm_mlo.txt. "The actual figures fluctuate month-by-month throughout the year, so figures for the same month of different years should be compared, or a seasonally corrected figure used."
- ↑ "Conversion Tables". Oak Ridge National Laboratory. 18 July 2020. https://cdiac.ess-dive.lbl.gov/pns/convert.html. Alt URL
- ↑ 23.0 23.1 23.2 Eyring, V., N.P. Gillett, K.M. Achuta Rao, R. Barimalala, M. Barreiro Parrillo, N. Bellouin, C. Cassou, P.J. Durack, Y. Kosaka, S. McGregor, S. Min, O. Morgenstern, and Y. Sun, 2021: Chapter 3: Human Influence on the Climate System . In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 423–552, doi:10.1017/9781009157896.005.
- ↑ "The World Counts". https://www.theworldcounts.com/challenges/climate-change/global-warming/global-co2-emissions.
- ↑ Rasmussen, Carl Edward. "Atmospheric Carbon Dioxide Growth Rate". http://mlg.eng.cam.ac.uk/carl/words/carbon.html.
- ↑ "Frequently Asked Questions". Carbon Dioxide Information Analysis Center (CDIAC). http://cdiac.ornl.gov/pns/faq.html.
- ↑ "Elevated atmospheric CO2 concentration and temperature across an urban–rural transect". Atmospheric Environment 41 (35): 7654–7665. 2007. doi:10.1016/j.atmosenv.2007.08.018. Bibcode: 2007AtmEn..41.7654G. https://zenodo.org/record/1258774. Retrieved 12 September 2019.
- ↑ 28.0 28.1 "IPCC: Climate Change 2001: The Scientific Basis". https://www.ipcc.ch/site/assets/uploads/2018/07/WG1_TAR_FM.pdf.
- ↑ 29.0 29.1 Tans, Pieter. "Trends in Carbon Dioxide". NOAA/ESRL. http://www.esrl.noaa.gov/gmd/ccgg/trends/.
- ↑ "Carbon Budget 2009 Highlights". globalcarbonproject.org. http://www.globalcarbonproject.org/carbonbudget/09/hl-full.htm.
- ↑ "Carbon dioxide passes symbolic mark". BBC. 10 May 2013. https://www.bbc.co.uk/news/science-environment-22486153.
- ↑ "Up-to-date weekly average CO2 at Mauna Loa". NOAA. http://www.esrl.noaa.gov/gmd/ccgg/trends/weekly.html.
- ↑ "Greenhouse gas levels pass symbolic 400ppm CO2 milestone". The Guardian. Associated Press. 1 June 2012. https://www.theguardian.com/environment/2012/jun/01/record-greenhouse-gas-trouble-scientists.
- ↑ Kunzig, Robert (2013-05-09). "Climate Milestone: Earth's CO2 Level Passes 400 ppm". National Geographic. http://news.nationalgeographic.com/news/energy/2013/05/130510-earth-co2-milestone-400-ppm/.
- ↑ 35.0 35.1 "Trends in Atmospheric Carbon Dioxide". NOAA. https://www.esrl.noaa.gov/gmd/ccgg/trends/.
- ↑ "The Early Keeling Curve | Scripps CO
2 Program". https://scrippsco2.ucsd.edu/history_legacy/early_keeling_curve.html. - ↑ "NOAA CCGG page Retrieved 2 March 2016". http://www.esrl.noaa.gov/gmd/ccgg/index.html.
- ↑ WDCGG webpage Retrieved 2 March 2016
- ↑ RAMCES webpage [yes|permanent dead link|dead link}}] Retrieved 2 March 2016
- ↑ "CDIAC CO2 page Retrieved 9 February 2016". http://cdiac.ornl.gov/trends/co2/.
- ↑ "GLOBALVIEW-CO2 information page. Retrieved 9 February 2016". http://www.esrl.noaa.gov/gmd/ccgg/globalview/co2/co2_intro.html.
- ↑ "TCCON data use policy webpage Retrieved 9 February 2016". https://tccon-wiki.caltech.edu/Network_Policy/Data_Use_Policy.
- ↑ e.g. Gosh, Prosenjit; Brand, Willi A. (2003). "Stable isotope ratio mass spectrometry in global climate change research". International Journal of Mass Spectrometry 228 (1): 1–33. doi:10.1016/S1387-3806(03)00289-6. Bibcode: 2003IJMSp.228....1G. http://www.bgc.mpg.de/service/iso_gas_lab/publications/PG_WB_IJMS.pdf. Retrieved 2 July 2012. "Global change issues have become significant due to the sustained rise in atmospheric trace gas concentrations (CO
2, N2O, CH4) over recent years, attributable to the increased per capita energy consumption of a growing global population.". - ↑ Keeling, Charles D.; Piper, Stephen C.; Whorf, Timothy P.; Keeling, Ralph F. (2011). "Evolution of natural and anthropogenic fluxes of atmospheric CO2 from 1957 to 2003". Tellus B 63 (1): 1–22. doi:10.1111/j.1600-0889.2010.00507.x. ISSN 0280-6509. Bibcode: 2011TellB..63....1K.
- ↑ Bender, Michael L.; Ho, David T.; Hendricks, Melissa B.; Mika, Robert; Battle, Mark O.; Tans, Pieter P.; Conway, Thomas J.; Sturtevant, Blake et al. (2005). "Atmospheric O2/N2changes, 1993–2002: Implications for the partitioning of fossil fuel CO2sequestration". Global Biogeochemical Cycles 19 (4): n/a. doi:10.1029/2004GB002410. ISSN 0886-6236. Bibcode: 2005GBioC..19.4017B.
- ↑ Evans, Simon (5 October 2021). "Analysis: Which countries are historically responsible for climate change? / Historical responsibility for climate change is at the heart of debates over climate justice.". Carbon Brief. https://www.carbonbrief.org/analysis-which-countries-are-historically-responsible-for-climate-change. "Source: Carbon Brief analysis of figures from the Global Carbon Project, CDIAC, Our World in Data, Carbon Monitor, Houghton and Nassikas (2017) and Hansis et al (2015)."
- ↑ Ballantyne, A.P.; Alden, C.B.; Miller, J.B.; Tans, P.P.; White, J.W.C. (2012). "Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years". Nature 488 (7409): 70–72. doi:10.1038/nature11299. ISSN 0028-0836. PMID 22859203. Bibcode: 2012Natur.488...70B.
- ↑ 48.0 48.1 Friedlingstein, P., Jones, M., O'Sullivan, M., Andrew, R., Hauck, J., Peters, G., Peters, W., Pongratz, J., Sitch, S., Le Quéré, C. and 66 others (2019) "Global carbon budget 2019". Earth System Science Data, 11(4): 1783–1838. doi:10.5194/essd-11-1783-2019.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Dlugokencky, E. (5 February 2016). "Annual Mean Carbon Dioxide Data". NOAA. ftp://aftp.cmdl.noaa.gov/products/trends/co2/co2_annmean_gl.txt.
- ↑ A.P. Ballantyne; C.B. Alden; J.B. Miller; P.P. Tans; J.W. C. White (2012). "Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years". Nature 488 (7409): 70–72. doi:10.1038/nature11299. PMID 22859203. Bibcode: 2012Natur.488...70B.
- ↑ 51.0 51.1 "Global carbon budget 2010 (summary)". Tyndall Centre for Climate Change Research. http://www.tyndall.ac.uk/global-carbon-budget-2010.
- ↑ Calculated from file global.1751_2013.csv in [1] from the Carbon Dioxide Information Analysis Center.
- ↑ IEA (2023), The world’s top 1% of emitters produce over 1000 times more CO
2 than the bottom 1%, IEA, Paris https://www.iea.org/commentaries/the-world-s-top-1-of-emitters-produce-over-1000-times-more-co2-than-the-bottom-1 , License: CC BY 4.0 - ↑ "Annex II Glossary". Intergovernmental Panel on Climate Change. http://www.ipcc.ch/publications_and_data/ar4/syr/en/annexessglossary-e-i.html.
- ↑ A concise description of the greenhouse effect is given in the Intergovernmental Panel on Climate Change Fourth Assessment Report, "What is the Greenhouse Effect?" FAQ 1.3 – AR4 WGI Chapter 1: Historical Overview of Climate Change Science , IPCC Fourth Assessment Report, Chapter 1, p. 115: "To balance the absorbed incoming [solar] energy, the Earth must, on average, radiate the same amount of energy back to space. Because the Earth is much colder than the Sun, it radiates at much longer wavelengths, primarily in the infrared part of the spectrum (see Figure 1). Much of this thermal radiation emitted by the land and ocean is absorbed by the atmosphere, including clouds, and reradiated back to Earth. This is called the greenhouse effect."
Stephen H. Schneider, in Geosphere-biosphere Interactions and Climate, Lennart O. Bengtsson and Claus U. Hammer, eds., Cambridge University Press, 2001, ISBN:0-521-78238-4, pp. 90–91.
E. Claussen, V.A. Cochran, and D.P. Davis, Climate Change: Science, Strategies, & Solutions, University of Michigan, 2001. p. 373.
A. Allaby and M. Allaby, A Dictionary of Earth Sciences, Oxford University Press, 1999, ISBN:0-19-280079-5, p. 244. - ↑ Vaclav Smil (2003). The Earth's Biosphere: Evolution, Dynamics, and Change. MIT Press. p. 107. ISBN 978-0-262-69298-4. https://books.google.com/books?id=8ntHWPMUgpMC&pg=PA107. Retrieved 14 March 2023.
- ↑ "Solar Radiation and the Earth's Energy Balance". The Climate System – EESC 2100 Spring 2007. Columbia University. http://eesc.columbia.edu/courses/ees/climate/lectures/radiation/.
- ↑ "Historical Overview of Climate Change Science". Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY: Cambridge University Press. 2007. p. 97. http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter1.pdf. Retrieved 25 March 2014.
- ↑ "The Elusive Absolute Surface Air Temperature (SAT)". NOAA. http://data.giss.nasa.gov/gistemp/abs_temp.html.
- ↑ "IPCC Fifth Assessment Report – Chapter 8: Anthropogenic and Natural Radiative Forcing.". https://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter08_FINAL.pdf.
- ↑ Arrhenius, Svante (1896). "On the influence of carbonic acid in the air upon the temperature of the ground". Philosophical Magazine and Journal of Science: 237–76. http://www.rsc.org/images/Arrhenius1896_tcm18-173546.pdf. Retrieved 14 March 2023.
- ↑ Riebeek, Holli (16 June 2011). "The Carbon Cycle". NASA. http://earthobservatory.nasa.gov/Features/CarbonCycle/?src=eoa-features.
- ↑ Kayler, Z.; Janowiak, M.; Swanston, C. (2017). "The Global Carbon Cycle". Considering Forest and Grassland Carbon in Land Management. United States Department of Agriculture, Forest Service. 3–9. https://www.fs.fed.us/research/publications/gtr/gtr_wo95.pdf. Retrieved 14 March 2023.
- ↑ Gerlach, T.M. (4 June 1991). "Present-day CO2 emissions from volcanoes". Eos, Transactions, American Geophysical Union 72 (23): 249, 254–55. doi:10.1029/90EO10192. Bibcode: 1991EOSTr..72..249..
- ↑ Cappelluti, G.; Bösch, H.; Monks, P.S. (2009). Use of remote sensing techniques for the detection and monitoring of GHG emissions from the Scottish land use sector. Scottish Government. ISBN 978-0-7559-7738-3. http://www.scotland.gov.uk/Publications/2009/12/15084401/0. Retrieved 28 January 2011.
- ↑ 66.0 66.1 66.2 Junling Huang; Michael B. McElroy (2012). "The Contemporary and Historical Budget of Atmospheric CO2". Canadian Journal of Physics 90 (8): 707–16. doi:10.1139/p2012-033. Bibcode: 2012CaJPh..90..707H. http://dash.harvard.edu/bitstream/handle/1/10981610/The%20contemporary%20and%20historical%20budget%20of%20atmospheric%20CO2_1%202.pdf?sequence=9. Retrieved 14 March 2023.
- ↑ "Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks". Proc. Natl. Acad. Sci. U.S.A. 104 (47): 18866–70. November 2007. doi:10.1073/pnas.0702737104. PMID 17962418. Bibcode: 2007PNAS..10418866C.
- ↑ "Historical Variations in Terrestrial Biospheric Carbon Storage". DOE Research Summary 34 (1): 99–109. June 1997. doi:10.1029/96GB03942. Bibcode: 1997GBioC..11...99P. http://cdiac.esd.ornl.gov/pns/doers/doer34/doer34.htm. Retrieved 28 May 2011.
- ↑ "Life: past, present and future". Philos. Trans. R. Soc. Lond. B Biol. Sci. 354 (1392): 1923–39. December 1999. doi:10.1098/rstb.1999.0532. PMID 10670014.
- ↑ Whitmarsh J, Govindjee (1999). "The photosynthetic process". in Singhal GS. Concepts in photobiology: photosynthesis and photomorphogenesis. Boston: Kluwer Academic Publishers. pp. 11–51. ISBN 978-0-7923-5519-9. http://www.life.illinois.edu/govindjee/paper/gov.html#80. Retrieved 20 March 2014. "100 x 1015 grams of carbon/year fixed by photosynthetic organisms which is equivalent to 4 x 1018 kJ/yr = 4 x 1021J/yr of free energy stored as reduced carbon; (4 x 1018 kJ/yr) / (31,556,900 sec/yr) = 1.27 x 1014 J/yr; (1.27 x 1014 J/yr) / (1012 J/sec / TW) = 127 TW."
- ↑ Sustainable development and innovation in the energy sector. Berlin: Springer. 2005. p. 32. ISBN 978-3-540-23103-5. https://books.google.com/books?id=duVJsAqXlkEC&pg=PA32. Retrieved 2023-03-14. "The average global rate of photosynthesis is 130 TW (1 TW = 1 terawatt = 1012 watt)."
- ↑ "World Consumption of Primary Energy by Energy Type and Selected Country Groups, 1980–2004" (XLS). Energy Information Administration. 31 July 2006. http://www.eia.doe.gov/pub/international/iealf/table18.xls.
- ↑ "Primary production of the biosphere: integrating terrestrial and oceanic components". Science 281 (5374): 237–40. July 1998. doi:10.1126/science.281.5374.237. PMID 9657713. Bibcode: 1998Sci...281..237F. http://www.escholarship.org/uc/item/9gm7074q. Retrieved 2023-03-14.
- ↑ "Photosynthesis". McGraw-Hill Encyclopedia of Science & Technology. 13. New York: McGraw-Hill. 2007. ISBN 978-0-07-144143-8.
- ↑ "Prokaryotic photosynthesis and phototrophy illuminated". Trends Microbiol. 14 (11): 488–96. November 2006. doi:10.1016/j.tim.2006.09.001. PMID 16997562.
- ↑ 76.0 76.1 Susan Solomon; Gian-Kasper Plattner; Reto Knutti; Pierre Friedlingstein (February 2009). "Irreversible climate change due to carbon dioxide emissions". Proc. Natl. Acad. Sci. USA 106 (6): 1704–09. doi:10.1073/pnas.0812721106. PMID 19179281. Bibcode: 2009PNAS..106.1704S.
- ↑ Archer, David; Eby, Michael; Brovkin, Victor; Ridgwell, Andy; Cao, Long; Mikolajewicz, Uwe; Caldeira, Ken; Matsumoto, Katsumi et al. (2009). "Atmospheric Lifetime of Fossil Fuel Carbon Dioxide". Annual Review of Earth and Planetary Sciences 37 (1): 117–34. doi:10.1146/annurev.earth.031208.100206. ISSN 0084-6597. Bibcode: 2009AREPS..37..117A. http://orbi.ulg.ac.be/handle/2268/12933. Retrieved 14 March 2023.
- ↑ Keeling, Charles D. (1997-08-05). "Climate change and carbon dioxide: An introduction" (in en). Proceedings of the National Academy of Sciences 94 (16): 8273–8274. doi:10.1073/pnas.94.16.8273. ISSN 0027-8424. PMID 11607732. Bibcode: 1997PNAS...94.8273K.
- ↑ Pisoft, Petr (May 25, 2021). "Stratospheric contraction caused by increasing greenhouse gases". Environmental Research Letters 16 (6): 064038. doi:10.1088/1748-9326/abfe2b. Bibcode: 2021ERL....16f4038P.
- ↑ Walker, James C.G. (June 1985). "Carbon dioxide on the early earth". Origins of Life and Evolution of the Biosphere 16 (2): 117–27. doi:10.1007/BF01809466. PMID 11542014. Bibcode: 1985OrLi...16..117W. http://deepblue.lib.umich.edu/bitstream/2027.42/43349/1/11084_2005_Article_BF01809466.pdf. Retrieved 2010-01-30.
- ↑ Pavlov, Alexander A.; Kasting, James F.; Brown, Lisa L.; Rages, Kathy A.; Freedman, Richard (May 2000). "Greenhouse warming by CH4 in the atmosphere of early Earth". Journal of Geophysical Research 105 (E5): 11981–90. doi:10.1029/1999JE001134. PMID 11543544. Bibcode: 2000JGR...10511981P.
- ↑ Zahnle, K.; Schaefer, L.; Fegley, B. (2010). "Earth's Earliest Atmospheres". Cold Spring Harbor Perspectives in Biology 2 (10): a004895. doi:10.1101/cshperspect.a004895. PMID 20573713.
- ↑ Olson JM (May 2006). "Photosynthesis in the Archean era". Photosynth. Res. 88 (2): 109–17. doi:10.1007/s11120-006-9040-5. PMID 16453059. Bibcode: 2006PhoRe..88..109O.
- ↑ Buick R (August 2008). "When did oxygenic photosynthesis evolve?". Philos. Trans. R. Soc. Lond. B Biol. Sci. 363 (1504): 2731–43. doi:10.1098/rstb.2008.0041. PMID 18468984.
- ↑ 85.0 85.1 85.2 Osborne, C.P.; Beerling, D.J. (2006). "Nature's green revolution: the remarkable evolutionary rise of C4 plants". Philosophical Transactions of the Royal Society B: Biological Sciences 361 (1465): 173–94. doi:10.1098/rstb.2005.1737. PMID 16553316.
- ↑ Lovelock, J. E. (1972). "Gaia as seen through the atmosphere". Atmospheric Environment 6 (8): 579–580. doi:10.1016/0004-6981(72)90076-5. Bibcode: 1972AtmEn...6..579L. http://www.jameslovelock.org/page33.html/. Retrieved 2014-03-22.
- ↑ Li, K.-F. (2009-05-30). "Atmospheric pressure as a natural climate regulator for a terrestrial planet with a biosphere". Proceedings of the National Academy of Sciences 106 (24): 9576–9579. doi:10.1073/pnas.0809436106. PMID 19487662. PMC 2701016. Bibcode: 2009PNAS..106.9576L. http://www.pnas.org/content/early/2009/06/01/0809436106.full.pdf+html. Retrieved 2014-03-22.
- ↑ Etheridge, D.M.; Steele, L.P.; Langenfelds, R.L.; Francey, R.J.; Barnola, JM; Morgan, VI (June 1998). "Historical CO2 record derived from a spline fit (20-year cutoff) of the Law Dome DE08 and DE08-2 ice cores". Oak Ridge National Laboratory. http://cdiac.ornl.gov/ftp/trends/co2/lawdome.smoothed.yr20.
- ↑ Flückiger, Jacqueline (2002). "High-resolution Holocene N2O ice core record and its relationship with CH4 and CO2". Global Biogeochemical Cycles 16 (1): 1010. doi:10.1029/2001GB001417. Bibcode: 2002GBioC..16.1010F.
- ↑ Amos, J. (4 September 2006). "Deep ice tells long climate story". BBC News. http://news.bbc.co.uk/2/hi/science/nature/5314592.stm.
- ↑ Hileman B. (November 2005). "Ice Core Record Extended: Analyses of trapped air show current CO2 at highest level in 650,000 years". Chemical & Engineering News 83 (48): 7. doi:10.1021/cen-v083n048.p007. ISSN 0009-2347. http://pubs.acs.org/cen/news/83/i48/8348notw1.html. Retrieved 28 January 2010.
- ↑ Vostok Ice Core Data , ncdc.noaa.gov
- ↑ Friederike Wagner; Bent Aaby; Henk Visscher (2002). "Rapid atmospheric CO2 changes associated with the 8,200-years-B.P. cooling event". Proc. Natl. Acad. Sci. USA 99 (19): 12011–14. doi:10.1073/pnas.182420699. PMID 12202744. Bibcode: 2002PNAS...9912011W.
- ↑ Andreas Indermühle; Bernhard Stauffer; Thomas F. Stocker (1999). "Early Holocene Atmospheric CO2 Concentrations". Science 286 (5446): 1815. doi:10.1126/science.286.5446.1815a. IndermÜhle, A (1999). "Early Holocene atmospheric CO2concentrations". Science 286 (5446): 1815a–15. doi:10.1126/science.286.5446.1815a.
- ↑ H. J. Smith; M. Wahlen; D. Mastroianni (1997). "The CO2 concentration of air trapped in GISP2 ice from the Last Glacial Maximum-Holocene transition". Geophysical Research Letters 24 (1): 1–4. doi:10.1029/96GL03700. Bibcode: 1997GeoRL..24....1S.
- ↑ File:Phanerozoic Carbon Dioxide.png
- ↑ 97.0 97.1 Witkowski, Caitlyn (28 November 2018). "Molecular fossils from phytoplankton reveal secular pCO2 trend over the Phanerozoic". Science Advances 2 (11): eaat4556. doi:10.1126/sciadv.aat4556. PMID 30498776. Bibcode: 2018SciA....4.4556W.
- ↑ Berner, Robert A. (January 1994). "GEOCARB II: a revised model of atmospheric CO2 over Phanerozoic time". American Journal of Science 294 (1): 56–91. doi:10.2475/ajs.294.1.56. Bibcode: 1994AmJS..294...56B.
- ↑ Royer, D.L.; R.A. Berner; D.J. Beerling (2001). "Phanerozoic atmospheric CO2 change: evaluating geochemical and paleobiological approaches". Earth-Science Reviews 54 (4): 349–92. doi:10.1016/S0012-8252(00)00042-8. Bibcode: 2001ESRv...54..349R.
- ↑ Berner, Robert A.; Kothavala, Zavareth (2001). "GEOCARB III: a revised model of atmospheric CO2 over Phanerozoic time". American Journal of Science 301 (2): 182–204. doi:10.2475/ajs.301.2.182. Bibcode: 2001AmJS..301..182B. https://earth.geology.yale.edu/~ajs/2001/Feb/qn020100182.pdf.
- ↑ Beerling, D.J.; Berner, R.A. (2005). "Feedbacks and the co-evolution of plants and atmospheric CO2". Proc. Natl. Acad. Sci. USA 102 (5): 1302–05. doi:10.1073/pnas.0408724102. PMID 15668402. Bibcode: 2005PNAS..102.1302B.
- ↑ 102.0 102.1 Hoffmann, PF; AJ Kaufman; GP Halverson; DP Schrag (1998). "A neoproterozoic snowball earth". Science 281 (5381): 1342–46. doi:10.1126/science.281.5381.1342. PMID 9721097. Bibcode: 1998Sci...281.1342H.
- ↑ Siegel, Ethan. "How Much CO2 Does A Single Volcano Emit?" (in en). Forbes. https://www.forbes.com/sites/startswithabang/2017/06/06/how-much-co2-does-a-single-volcano-emit/.
- ↑ Gerlach, TM (1991). "Present-day CO2 emissions from volcanoes". Transactions of the American Geophysical Union 72 (23): 249–55. doi:10.1029/90EO10192. Bibcode: 1991EOSTr..72..249..
- ↑ See also: "U.S. Geological Survey". 14 June 2011. http://www.usgs.gov/newsroom/article.asp?ID=2827&from=rss_home.
- ↑ "New CO2 data helps unlock the secrets of Antarctic formation". Physorg.com. 13 September 2009. http://www.physorg.com/news172072921.html.
- ↑ Pagani, Mark; Huber, Matthew; Liu, Zhonghui; Bohaty, Steven M.; Henderiks, Jorijntje; Sijp, Willem; Krishnan, Srinath; Deconto, Robert M. (2 December 2011). "Drop in carbon dioxide levels led to polar ice sheet, study finds". Science 334 (6060): 1261–4. doi:10.1126/science.1203909. PMID 22144622. Bibcode: 2011Sci...334.1261P. https://www.sciencedaily.com/releases/2011/12/111201174225.htm. Retrieved 14 May 2013.
External links
- Current global map of carbon dioxide concentrations.
- Global Carbon Dioxide Circulation (NASA; 13 December 2016)
- Video (03:10) – A Year in the Life of Earth's CO
2 (NASA; 17 November 2014)
fr:Dioxyde de carbone#CO2 dans l'atmosphère terrestre
 |