Chemistry:Sulfonyl group
From HandWiki
Short description: Chemical group (>S(=O)₂)
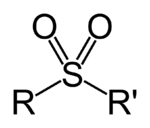
In organosulfur chemistry, a sulfonyl group can refer either to a functional group found primarily in sulfones, or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups.[1]:1470-1476 Sulfonyl groups can be written as having the general formula R–S(=O)
2–R′, where there are two double bonds between the sulfur and oxygen.[1]:53[2]
Sulfonyl groups can be reduced to the sulfide with DIBALH. Lithium aluminium hydride (LiAlH
4) reduces some but not all sulfones to sulfides.[1]:1851
In inorganic chemistry, when the group –S(=O)
2– is not connected to any carbon atoms, it is referred to as sulfuryl.[3]
Examples of sulfonyl group substituents
The names of sulfonyl groups typically end in -syl, such as:[1]:497
Group name Full name Pseudoelement symbol Example Tosyl p-toluenesulfonyl Ts Tosyl chloride (p-toluenesulfonyl chloride)
CH3C6H4SO2ClBrosyl p-bromobenzenesulfonyl Bs Nosyl o- or p-nitrobenzenesulfonyl Ns Mesyl methanesulfonyl Ms Mesyl chloride (methanesulfonyl chloride)
CH3SO2ClTriflyl trifluoromethanesulfonyl Tf Tresyl 2,2,2-trifluoroethyl-1-sulfonyl Dansyl 5-(dimethylamino)naphthalene-1-sulfonyl Ds Dansyl chloride
See also
References
- ↑ 1.0 1.1 1.2 1.3 Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). John Wiley & Sons. ISBN 978-0-471-72091-1.
- ↑ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. pp. 1249-1251. ISBN 978-0-19-850346-0.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 694-695. ISBN 978-0-08-037941-8.
 |