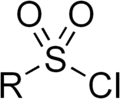Chemistry:Sulfonyl halide
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl (>S(=O)
2) functional group is singly bonded to a halogen atom. They have the general formula RSO
2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.[1][2]
Structure
Sulfonyl halides have tetrahedral sulfur centres attached to two oxygen atoms, an organic radical, and a halide. In a representative example, methanesulfonyl chloride, the S=O, S−C, and S−Cl bond distances are respectively 142.4, 176.3, and 204.6 pm.[3]
Sulfonyl chlorides
Sulfonic acid chlorides, or sulfonyl chlorides, are a sulfonyl halide with the general formula RSO
2Cl.
Production
Arylsulfonyl chlorides are made industrially in a two-step, one-pot reaction from an arene (in this case, benzene) and chlorosulfuric acid:[4]
- [math]\ce{ C6H6 + HOSO2Cl -> C6H5SO3H + HCl }[/math]
- [math]\ce{ C6H5SO3H + HOSO2Cl -> C6H5SO2Cl + H2SO4 }[/math]
The intermediate benzenesulfonic acid can be chlorinated with thionyl chloride as well. Benzenesulfonyl chloride, the most important sulfonyl halide, can also be produced by treating sodium benzenesulfonate with phosphorus pentachlorides.[5]
Benzenediazonium chloride reacts with sulfur dioxide and hydrochloric acid to give the sulfonyl chloride:
- [math]\ce{ [C6H5N2]Cl + SO2 -> C6H5SO2Cl + N2 }[/math]
For alkylsulfonyl chlorides, one synthetic procedure is the Reed reaction:
- [math]\ce{ RH + SO2 + Cl2 -> RSO2Cl + HCl }[/math]
Reactions
Sulfonyl chlorides react with water to give the corresponding sulfonic acid:
- [math]\ce{ RSO2Cl + H2O -> RSO3H + HCl }[/math]
These compounds react readily with many other nucleophiles as well, most notably alcohols and amines (see Hinsberg reaction). If the nucleophile is an alcohol, the product is a sulfonate ester; if it is an amine, the product is a sulfonamide. Using sodium sulfite as the nucleophilic reagent, p-toluenesulfonyl chloride is converted to its sulfinate salt, CH
3C
6H
4SO
2Na.[6] Chlorosulfonated alkanes are susceptible to crosslinking via reactions with various nucleophiles.[7]
Sulfonyl chlorides readily undergo Friedel–Crafts reactions with arenes giving sulfones, for example:
- [math]\ce{ RSO2Cl + C6H6 -> RSO2C6H5 + HCl }[/math]
The desulfonation of arylsulfonyl chlorides provides a route to aryl chlorides:
- [math]\ce{ ArSO2Cl -> ArCl + SO2 }[/math]
1,2,4-Trichlorobenzene is made industrially in this way.
Treatment of alkanesulfonyl chlorides having α-hydrogens with amine bases can give sulfenes, highly unstable species that can be trapped:
- [math]\ce{ RCH2SO2Cl -> RCH=SO2 }[/math]
Common sulfonyl chlorides
Chlorosulfonated polyethylene (CSPE) is produced industrially by chlorosulfonation of polyethylene. CSPE is noted for its toughness, hence its use for roofing shingles.[7]
An industrially important derivative is benzenesulfonyl chloride. In the laboratory, useful reagents include tosyl chloride, brosyl chloride, nosyl chloride and mesyl chloride.
Sulfonyl fluorides
Sulfonyl fluorides have the general formula RSO2F. "Most, if not all" industrially synthesized perfluorooctanesulfonyl derivatives, such as PFOS, have the sulfonyl fluoride as their precursor.[8]
In the laboratory, sulfonyl fluorides are used in molecular biology as reactive probes. They specifically react with residues based on serine, threonine, tyrosine, lysine, cysteine, and histidine. The fluorides are more resistant than the corresponding chlorides and are therefore better suited to this task.[9]
Some sulfonyl fluorides can also be used as deoxyfluorinating reagents, such as 2-pyridinesulfonyl fluoride (PyFluor) and N-tosyl-4-chlorobenzenesulfonimidoyl fluoride (SulfoxFluor).[10][11]
Sulfonyl bromides
Sulfonyl bromides have the general formula RSO2Br. In contrast to sulfonyl chlorides, sulfonyl bromides readily undergo light-induced homolysis affording sulfonyl radicals, which can add to alkenes, as illustrated by the use of bromomethanesulfonyl bromide, BrCH2SO2Br in Ramberg–Bäcklund reaction syntheses.[12][13]
Sulfonyl iodides
Sulfonyl iodides, having the general formula RSO2I, are quite light-sensitive. Perfluoroalkanesulfonyl iodides, prepared by reaction between silver perfluoroalkanesulfinates and iodine in dichloromethane at −30 °C, react with alkenes to form the normal adducts, RFSO2CH2CHIR and the adducts resulting from loss of SO2, RFCH2CHIR.[14] Arenesulfonyl iodides, prepared from reaction of arenesulfinates or arenehydrazides with iodine, can be used as initiators to facilitate the synthesis of poly(methyl methacrylate) containing C–I, C–Br and C–Cl chain ends.[15]
In popular culture
In the episode "Encyclopedia Galactica" of his TV series Cosmos: A Personal Voyage, Carl Sagan speculates that some intelligent extraterrestrial beings might have a genetic code based on polyaromatic sulfonyl halides instead of DNA.
References
- ↑ Kosswig, Kurt (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_503.
- ↑ Drabowicz, J.; Kiełbasiński, P.; Łyżwa, P.; Zając, A.; Mikołajczyk, M. (2008). N. Kambe. ed. Alkanesulfonyl Halides. Science of Synthesis. 39. pp. 19–38. ISBN 9781588905307.
- ↑ Hargittai, Magdolna; Hargittai, István (1973). "On the molecular structure of methane sulfonyl chloride as studied by electron diffraction". J. Chem. Phys. 59 (5): 2513. doi:10.1063/1.1680366. Bibcode: 1973JChPh..59.2513H.
- ↑ Lindner, Otto; Rodefeld, Lars. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_507.
- ↑ Adams, Roger; Marvel, C. S.; Clarke, H. T.; Babcock, G. S.; Murray, T. F. (1921). "Benzenesulfonyl chloride". Organic Syntheses 1: 21. http://www.orgsyn.org/demo.aspx?prep=CV1P0084.; Collective Volume, 1, pp. 84
- ↑ Field, L; Clark, R.D. (1958). "Methyl p-Tolyl Sulfone". Organic Syntheses 38: 62. doi:10.15227/orgsyn.038.0062. http://orgsyn.org/demo.aspx?prep=CV4P0674. Retrieved 9 July 2023.
- ↑ 7.0 7.1 Happ, Michael; Duffy, John; Wilson, G. J.; Pask, Stephen D.; Buding, Hartmuth; Ostrowicki, Andreas (2011). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o23_o05.
- ↑ Lehmler, H. J. (2005). "Synthesis of environmentally relevant fluorinated surfactants—a review". Chemosphere 58 (11): 1471–1496. doi:10.1016/j.chemosphere.2004.11.078. PMID 15694468. Bibcode: 2005Chmsp..58.1471L.
- ↑ Narayanan, Arjun; Jones, Lyn H. (2015). "Sulfonyl fluorides as privileged warheads in chemical biology". Chemical Science 6 (5): 2650–2659. doi:10.1039/C5SC00408J. PMID 28706662.
- ↑ Nielsen, Matthew K.; Ugaz, Christian R.; Li, Wenping; Doyle, Abigail G. (5 August 2015). "PyFluor: A Low-Cost, Stable, and Selective Deoxyfluorination Reagent". Journal of the American Chemical Society 137 (30): 9571–9574. doi:10.1021/jacs.5b06307.
- ↑ Guo, Junkai; Kuang, Cuiwen; Rong, Jian; Li, Lingchun; Ni, Chuanfa; Hu, Jinbo (28 May 2019). "Rapid Deoxyfluorination of Alcohols with N‐Tosyl‐4‐chlorobenzenesulfonimidoyl Fluoride (SulfoxFluor) at Room Temperature". Chemistry – A European Journal 25 (30): 7259–7264. doi:10.1002/chem.201901176.
- ↑ Block, E.; Aslam, M. (1993). "A General Synthetic Method for the Preparation of Conjugated Dienes from Olefins using Bromomethanesulfonyl Bromide: 1,2-Dimethylenecyclohexane". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv8p0212.; Collective Volume, Coll. Vol. 8, pp. 212
- ↑ Block, E.; Aslam, M.; Eswarakrishnan, V.; Gebreyes, K.; Hutchinson, J.; Iyer, R.; Laffitte, J.-A.; Wall, A. (1986). "α-Haloalkanesulfonyl Bromides in Organic Synthesis. 5. Versatile Reagents for the Synthesis of Conjugated Polyenes, Enones and 1,3-Oxathiole 1,1-Dioxides". J. Am. Chem. Soc. 108 (15): 4568–4580. doi:10.1021/ja00275a051.
- ↑ Huang, W.-Y.; L.-Q., Hu (1989). "The chemistry of perfluoroalkanesulfonyl iodides". Journal of Fluorine Chemistry 44 (1): 25–44. doi:10.1016/S0022-1139(00)84369-9.
- ↑ Percec, V.; Grigoras, C. (2005). "Arenesulfonyl iodides: The third universal class of functional initiators for the metal-catalyzed living radical polymerization of methacrylates and styrenes.". Journal of Polymer Science Part A: Polymer Chemistry 43 (17): 3920–3931. doi:10.1002/pola.20860. Bibcode: 2005JPoSA..43.3920P.
 |