Biology:Alpha-glucosidase
| α-glucosidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 α-Glucosidase hexamer, Sulfolobus solfataricus | |||||||||
| Identifiers | |||||||||
| EC number | 3.2.1.20 | ||||||||
| CAS number | 9001-42-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||

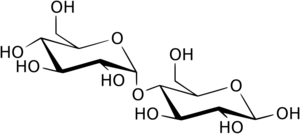
α-Glucosidase (EC 3.2.1.20, maltase, glucoinvertase, glucosidosucrase, maltase-glucoamylase, α-glucopyranosidase, glucosidoinvertase, α-D-glucosidase, α-glucoside hydrolase, α-1,4-glucosidase, α-D-glucoside glucohydrolase; systematic name α-D-glucoside glucohydrolase) is a glucosidase located in the brush border of the small intestine that acts upon α(1→4) bonds:[1][2][3][4][5][6]
- Hydrolysis of terminal, non-reducing (1→4)-linked α-D-glucose residues with release of D-glucose
This is in contrast to EC 3.2.1.21 β-glucosidase. α-Glucosidase breaks down starch and disaccharides to glucose.
Other glucosidases include:
Mechanism
α-Glucosidase hydrolyzes terminal non-reducing (1→4)-linked α-glucose residues to release a single α-glucose molecule.[7] α-Glucosidase is a carbohydrate-hydrolase that releases α-glucose as opposed to β-glucose. β-Glucose residues can be released by glucoamylase, a functionally similar enzyme. The substrate selectivity of α-glucosidase is due to subsite affinities of the enzyme's active site.[8] Two proposed mechanisms include a nucleophilic displacement and an oxocarbenium ion intermediate.[8]

- Rhodnius prolixus, a blood-sucking insect, forms hemozoin (Hz) during digestion of host hemoglobin. Hemozoin synthesis is dependent on the substrate binding site of α-glucosidase.[9]
- Trout liver α-glucosidases were extracted and characterized. It was shown that for one of the trout liver α-glucosidases maximum activity of the enzyme was increased by 80% during exercise in comparison to a resting trout. This change was shown to correlate to an activity increase for liver glycogen phosphorylase. It is proposed that α-glucosidase in the glucosidic path plays an important part in complementing the phosphorolytic pathway in the liver's metabolic response to energy demands of exercise.[10]
- Yeast and rat small intestinal α-glucosidases have been shown to be inhibited by several groups of flavonoids.[11]
Structure
α-Glucosidases can be divided, according to primary structure, into two families.[8] The gene coding for human lysosomal α-glucosidase is about 20 kb long and its structure has been cloned and confirmed.[12]
- Human lysosomal α-glucosidase has been studied for the significance of the Asp-518 and other residues in proximity of the enzyme's active site. It was found that substituting Asp-513 with Glu-513 interferes with posttranslational modification and intracellular transport of α-glucosidase's precursor. Additionally, the Trp-516 and Asp-518 residues have been deemed critical for the enzyme's catalytic functionality.[13]
- Kinetic changes in α-glucosidase have been shown to be induced by denaturants such as guanidinium chloride (GdmCl) and SDS solutions. These denaturants cause loss of activity and conformational change. A loss of enzyme activity occurs at much lower concentrations of denaturant than required for conformational changes. This leads to a conclusion that the enzyme's active site conformation is less stable than the whole enzyme conformation in response to the two denaturants.[14]
Disease relevance
- Glycogen storage disease type II, also called Pompe disease: a disorder in which α-glucosidase is deficient. In 2006, the drug alglucosidase alfa became the first released treatment for Pompe disease and acts as an analog to α-glucosidase.[15] Further studies of alglucosidase alfa revealed that iminosugars exhibit inhibition of the enzyme. It was found that one compound molecule binds to a single enzyme molecule. It was shown that 1-deoxynojirimycin (DNJ) would bind the strongest of the sugars tested and blocked the active site of the enzyme almost entirely. The studies enhanced knowledge of the mechanism by which α-glucosidase binds to imino sugars.[16]
- Diabetes: Acarbose, an α-glucosidase inhibitor, competitively and reversibly inhibits α-glucosidase in the intestines. This inhibition lowers the rate of glucose absorption through delayed carbohydrate digestion and extended digestion time. Acarbose may be able to prevent the development of diabetic symptoms.[17] Hence, α-glucosidase inhibitors (like acarbose) are used as anti-diabetic drugs in combination with other anti-diabetic drugs. Luteolin has been found to be a strong inhibitor of α-glucosidase. The compound can inhibit the enzyme up to 36% with a concentration of 0.5 mg/ml.[18] As of 2016, this substance is being tested in rats, mice and cell culture. Flavonoid analogues have been demonstrated with inhibition activity.[19]
- Azoospermia: Diagnosis of azoospermia has potential to be aided by measurement of α-glucosidase activity in seminal plasma. Activity in the seminal plasma corresponds to the functionality of the epididymis.[20]
- Antiviral agents: Many animal viruses possess an outer envelope composed of viral glycoproteins. These are often required for the viral life cycle and utilize cellular machinery for synthesis. Inhibitors of α-glucosidase show that the enzyme is involved in the pathway for N-glycans for viruses such as HIV and human hepatitis B virus (HBV). Inhibition of α-glucosidase can prevent fusion of HIV and secretion of HBV.[21]
See also
References
- ↑ alpha-Glucosidases at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Bruni, C.B.; Sica, V.; Auricchio, F.; Covelli, I. (1970). "Further kinetic and structural characterization of the lysosomal α-D-glucoside glucohydrolase from cattle liver". Biochim. Biophys. Acta 212 (3): 470–477. doi:10.1016/0005-2744(70)90253-6. PMID 5466143.
- ↑ Flanagan, P.R.; Forstner, G.G. (1978). "Purification of rat intestinal maltase/glucoamylase and its anomalous dissociation either by heat or by low pH". Biochem. J. 173 (2): 553–563. doi:10.1042/bj1730553. PMID 29602.
- ↑ Larner, J.; Lardy, H.; Myrback, K. (1960). "Other glucosidases". in Boyer, P.D.. The Enzymes. 4 (2nd ed.). New York: Academic Press. pp. 369–378.
- ↑ Sivikami, S.; Radhakrishnan, A.N. (1973). "Purification of rabbit intestinal glucoamylase by affinity chromatography on Sephadex G-200". Indian J. Biochem. Biophys. 10 (4): 283–284. PMID 4792946.
- ↑ Sørensen, S.H.; Norén, O.; Sjöström, H.; Danielsen, E.M. (1982). "Amphiphilic pig intestinal microvillus maltase/glucoamylase. Structure and specificity". Eur. J. Biochem. 126 (3): 559–568. doi:10.1111/j.1432-1033.1982.tb06817.x. PMID 6814909.
- ↑ "EC 3.2.1.20". ExPASy. http://enzyme.expasy.org/EC/3.2.1.20.
- ↑ 8.0 8.1 8.2 Chiba S (August 1997). "Molecular mechanism in α-glucosidase and glucoamylase". Biosci. Biotechnol. Biochem. 61 (8): 1233–9. doi:10.1271/bbb.61.1233. PMID 9301101.
- ↑ "α-Glucosidase promotes hemozoin formation in a blood-sucking bug: an evolutionary history". PLOS ONE 4 (9): e6966. 2009. doi:10.1371/journal.pone.0006966. PMID 19742319. Bibcode: 2009PLoSO...4.6966M.
- ↑ "Characterization of α-glucosidases from rainbow trout liver". Arch. Biochem. Biophys. 306 (1): 188–94. October 1993. doi:10.1006/abbi.1993.1499. PMID 8215402.
- ↑ "Inhibition of α-glucosidase and α-amylase by flavonoids". J. Nutr. Sci. Vitaminol. 52 (2): 149–53. April 2006. doi:10.3177/jnsv.52.149. PMID 16802696.
- ↑ Hoefsloot L; M Hoogeveen-Westerveld; A J Reuser; B A Oostra (1 December 1990). "Characterization of the human lysosomal α-glucosidase gene.". Biochem. J. 272 (2): 493–497. doi:10.1042/bj2720493. PMID 2268276.
- ↑ Hermans, Monique; Marian Kroos; Jos Van Beeumen; Ben Oostra; Arnold Reuser (25 July 1991). "Human Lysosomal a-Glucosidase Characterization of The Catalytic Site". The Journal of Biological Chemistry. 21 266 (21): 13507–13512. doi:10.1016/S0021-9258(18)92727-4. http://www.jbc.org/content/266/21/13507.full.pdf+html. Retrieved 1 March 2012.
- ↑ "Inhibition kinetics and the aggregation of α-glucosidase by different denaturants". Protein J. 28 (9–10): 448–56. December 2009. doi:10.1007/s10930-009-9213-0. PMID 19921411.
- ↑ "FDA Approves First Treatment for Pompe Disease". FDA News Release. FDA. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108645.htm.
- ↑ Yoshimizu, M.; Tajima, Y; Matsuzawa, F; Aikawa, S; Iwamoto, K; Kobayashi, T; Edmunds, T; Fujishima, K et al. (May 2008). "Binding parameters and thermodynamics of the interaction of imino sugars with a recombinant human acid α-glucosidase (alglucosidase alfa): insight into the complex formation mechanism.". Clin Chim Acta 391 (1–2): 68–73. doi:10.1016/j.cca.2008.02.014. PMID 18328816.
- ↑ Bischoff H (August 1995). "The mechanism of α-glucosidase inhibition in the management of diabetes". Clin Invest Med 18 (4): 303–11. PMID 8549017.
- ↑ "Inhibition of α-glucosidase and amylase by luteolin, a flavonoid". Biosci. Biotechnol. Biochem. 64 (11): 2458–61. November 2000. doi:10.1271/bbb.64.2458. PMID 11193416.
- ↑ "Synthesis of novel flavonoid alkaloids as α-glucosidase inhibitors". Bioorganic & Medicinal Chemistry 25 (20): 5355–64. November 2017. doi:10.1016/j.bmc.2017.07.055. PMID 28797772.
- ↑ "Seminal plasma α-glucosidase activity and male infertility". Hum. Reprod. 13 (3): 591–5. March 1998. doi:10.1093/humrep/13.3.591. PMID 9572418.
- ↑ Mehta, Anand; Zitzmann, Nicole; Rudd, Pauline M; Block, Timothy M; Dwek, Raymond A (23 June 1998). "α-Glucosidase inhibitors as potential broad based anti-viral agents". FEBS Letters 430 (1–2): 17–22. doi:10.1016/S0014-5793(98)00525-0. PMID 9678587.
de:Maltase-Glucoamylase
