Biology:Alpha-galactosidase
| α-galactosidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 α-Galactosidase tetramer, Mortierella vinacea | |||||||||
| Identifiers | |||||||||
| EC number | 3.2.1.22 | ||||||||
| CAS number | 9025-35-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
α-Galactosidase ( EC 3.2.1.22, α-GAL, α-GAL A; systematic name α-D-galactoside galactohydrolase) is a glycoside hydrolase enzyme that catalyses the following reaction:[1]
- Hydrolysis of terminal, non-reducing α-D-galactose residues in α-D-galactosides, including galactose oligosaccharides, galactomannans and galactolipids
It catalyzes many catabolic processes, including cleavage of glycoproteins, glycolipids, and polysaccharides.
The enzyme is encoded by the GLA gene.[2] Two recombinant forms of human α-galactosidase are called agalsidase α (INN) and agalsidase β (INN). A mold-derived form is the primary ingredient in gas relief supplements.
Function
This enzyme is a homodimeric glycoprotein that hydrolyses the terminal α-galactosyl moieties from glycolipids and glycoproteins. It predominantly hydrolyzes ceramide trihexoside, and it can catalyze the hydrolysis of melibiose into galactose and glucose.
Reaction mechanism

Disease relevance
Fabry disease
Signs and Symptoms
Defects in human α-GAL result in Fabry disease, a rare lysosomal storage disorder and sphingolipidosis that results from a failure to catabolize α-D-galactosyl glycolipid moieties.[6] Characteristic features include episodes of pain in hands and feet (acroparesthesia), dark red spots on skin (angiokeratoma), decreased sweating (hypohidrosis), decreased vision (corneal opacity), gastrointestinal problems, hearing loss, tinnitus, etc.. Complications may be life-threatening and may include progressive kidney damage, heart attack, and stroke. This disease may have late onset and only affect the heart or kidneys.[7]
Fabry disease is an X-linked disease, affecting 1 in 40,000 males. However, unlike other X-linked diseases, this condition also creates significant medical problems for females carrying only 1 copy of the defective GLA gene. These women may experience many classic symptoms of the disorder including cardiac and kidney problems. However, a small number of females carrying only one copy of the mutated GLA gene never shows any symptoms of Fabry disease.
Cause
Mutations to the GLA gene encoding α-GAL may result in complete loss of function of the enzyme. α-GAL is a lysosomal protein responsible for breaking down globotriaosylceramide, a fatty substance stored various types of cardiac and renal cells.[8] When globotriaosylceramide is not properly catabolized, it is accumulated in cells lining blood vessels in the skin, cells in the kidney, heart and nervous system. As a result, signs and symptoms of Fabry disease begin to manifest.[7]
Treatment
There are three treatment options for Fabry disease: recombinant enzyme replacement therapy, pharmacological chaperone therapy, and organ specific treatment.
Recombinant enzyme replacement therapy (RERT)
RERT was approved as a treatment for Fabry disease in the United States in 2003.[9][10][11]
Two recombinant enzyme replacement therapies are available to functionally compensate for α-galactosidase deficiency. Agalsidase α and β are both recombinant forms of the human α-galactosidase A enzyme and both have the same amino acid sequence as the native enzyme. Agalsidase α and β differ in the structures of their oligosaccharide side chains.[12]
In Fabry disease patients, 88% percent of patients develop IgG antibodies towards the injected recombinant enzyme, as it is foreign to their immune system. One suggested approach to solving this problem involves converting the paralogous enzyme α-NAGAL (NAGA) into one that has with α-GAL activity. Because patients still have a functional NAGA gene, their immune system will not produce NAGA antibodies.[13]
Agalsidase α
The pharmaceutical company Shire manufactures agalsidase alfa (INN) under the trade name Replagal as a treatment for Fabry disease,[14] and was granted marketing approval in the EU in 2001.[15] FDA approval was applied for the United States.[16] However, in 2012, Shire withdrew their application for approval in the United States citing that the agency will require additional clinical trials before approval.[17]
Agalsidase β
The pharmaceutical company Genzyme produces synthetic agalsidase β (INN) under the trade name Fabrazyme for treatment of Fabry disease. In 2009, contamination at Genzyme's Allston, Massachusetts plant caused a worldwide shortage of Fabrazyme, and supplies were rationed to patients at one-third the recommended dose. Some patients have petitioned to break the company's patent on the drug under the "march-in" provisions of the Bayh–Dole Act.[16]
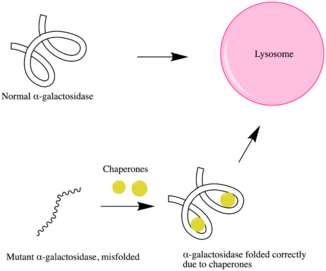
Pharmacological chaperone therapy
Fabry patients who display neurological symptoms cannot receive RERT because recombinant enzymes cannot normally pass the blood-brain barrier. Thus, a more suitable alternative treatment is used: pharmacological chaperone therapy.
It has been shown that more potent competitive inhibitors of an enzyme can act as a more powerful chemical chaperone for the corresponding mutant enzyme that fails to maintain proper folding and conformation, despite its intact active site. These chemical chaperones bind to the active site of the mutant enzyme, which can help promote proper folding and stabilize the mutant enzyme. Thus, this results in functional mutant enzymes that will not be degraded via the ubiquitin-proteasome pathway.
1-Deoxygalactonojirimycin (DGJ) has been shown to be both a potent competitive inhibitor of α-GAL and an effective chaperone to for Fabry disease, increasing intracellular α-GAL's activity by 14-fold.[20][21]
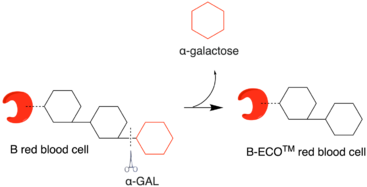
Modifying blood type group B to group O
α-GAL, known as B-zyme in this context, has also demonstrated its ability to convert human blood group B to human blood group O, which can be transfused to patients of all blood types in the ABO blood group categorization. The current B-zyme used comes from Bacteroides fragilis.[19] The idea of maintaining a blood supply at healthcare facilities with all non-O units converted to O units is achieved using enzyme-converted to group O technology, first developed in 1982.[22]
Advantages
A blood bank with ECO blood demonstrates the following advantages:[23]
- Compatible with and transfusable to patients of all blood groups
- Reduce the demand for specific ABO blood groups A, B, AB
- Reduce cost of maintaining a blood bank inventory in hospitals
- Reduce blood transfusion reactions due to human error and ABO incompatibility
- Reduce wastage of less needed blood types
Mechanism of Action
Red blood cell (RBC) surfaces are decorated with the glycoproteins and glycolipids that have the same basic sequence with terminal sugar α1‐2‐linked fucose linked to the penultimate galactose. This galactose molecule is called the H antigen.[24][25][26] Blood type A, B, AB, and O differ only in the sugar (red molecule in the illustration) linked with the penultimate galactose. For blood type B, this linked sugar is an α-1‐3‐linked galactose. Using α-GAL, this terminal galactose molecule can be removed, converting RBC to type O.
Supplements
α-GAL derived from the mold Aspergillus niger is an active ingredient in products marketed to reduce stomach gas production after eating foods known to cause gas. It is optimally active at 55°C, after which its half-life is 120 minutes.[27]
Commercial products with α-galactosidase include:
- Beano
- CVS BeanAid
- Enzymedica's BeanAssist
- Gasfix
- Bloateez (in India as Cogentrix)
See also
- β-galactosidase
- Migalastat, a drug targeting α-galactosidase
- Classification of α-galactosidases (according to CAZy)
References
- ↑ The Metabolic & Molecular Basis of Inherited Disease (8th ed.). McGraw-Hill. 15 December 2000. ISBN 978-0-07-913035-8.
- ↑ "Fabry disease: isolation of a cDNA clone encoding human α-galactosidase A". Proceedings of the National Academy of Sciences of the United States of America 82 (21): 7364–8. November 1985. doi:10.1073/pnas.82.21.7364. PMID 2997789. Bibcode: 1985PNAS...82.7364C.
- ↑ "Stereochemistry and the Mechanism of Enzymatic Reactions". Biological Reviews 28 (4): 416–436. 1953. doi:10.1111/j.1469-185x.1953.tb01386.x.
- ↑ "Lignocellulose degradation by Phanerochaete chrysosporium: purification and characterization of the main α-galactosidase". The Biochemical Journal 339 ( Pt 1) (1): 43–53. April 1999. doi:10.1042/bj3390043. PMID 10085226.
- ↑ "Mechanistic insights into glycosidase chemistry". Current Opinion in Chemical Biology 12 (5): 539–55. October 2008. doi:10.1016/j.cbpa.2008.05.010. PMID 18558099.
- ↑ "Entrez Gene: GLA galactosidase, alpha". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=2717.
- ↑ 7.0 7.1 Reference. "Fabry disease". Genetics Home Reference. https://ghr.nlm.nih.gov/condition/fabry-disease.
- ↑ Ronco, Claudio; Bellomo, Rinaldo; Bellasi, Antonio (2019). Critical Care Nephrology (Third ed.). Elsevier. pp. 704–711.e2. doi:10.1016/B978-0-323-44942-7.00115-1. ISBN 978-0-323-44942-7.
- ↑ "Enzyme replacement therapy in Fabry disease: a randomized controlled trial". JAMA 285 (21): 2743–9. June 2001. doi:10.1001/jama.285.21.2743. PMID 11386930.
- ↑ "Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease". The New England Journal of Medicine 345 (1): 9–16. July 2001. doi:10.1056/nejm200107053450102. PMID 11439963. http://dare.uva.nl/personal/pure/en/publications/safety-and-efficacy-of-recombinant-human-alphagalactosidase-a-replacement-therapy-in-fabrys-disease(b4b79813-d3b4-404f-b9b6-1d6e3952bfcc).html.
- ↑ "Enzyme replacement and enhancement therapies: lessons from lysosomal disorders". Nature Reviews. Genetics 3 (12): 954–66. December 2002. doi:10.1038/nrg963. PMID 12459725.
- ↑ "Safety and efficacy of enzyme replacement therapy in the nephropathy of Fabry disease". Biologics: Targets and Therapy 2 (4): 823–43. December 2008. doi:10.2147/btt.s3770. PMID 19707461.
- ↑ "Interconversion of the specificities of human lysosomal enzymes associated with Fabry and Schindler diseases". The Journal of Biological Chemistry 285 (28): 21560–6. July 2010. doi:10.1074/jbc.M110.118588. PMID 20444686.
- ↑ "Agalsidase alfa: a review of its use in the management of Fabry disease". BioDrugs 26 (5): 335–54. October 2012. doi:10.2165/11209690-000000000-00000. PMID 22946754.
- ↑ "Shire Submits Biologics License Application (BLA) for REPLAGAL with the U.S. Food and Drug Administration (FDA)". FierceBiotech. 22 December 2009. http://www.fiercebiotech.com/press-releases/shire-submits-biologics-license-application-bla-replagal-u-s-food-and-drug-administra.
- ↑ 16.0 16.1 "With A Life-Saving Medicine In Short Supply, Patients Want Patent Broken". 2010-08-04. https://www.npr.org/blogs/health/2010/08/04/128973687/with-a-life-saving-medicine-in-short-supply-patients-want-patent-broken.
- ↑ Grogan K (2012-03-15). "Shire withdraws Replagal in USA as FDA wants more trials". PharmaTimes. http://www.pharmatimes.com/article/12-03-15/Shire_withdraws_Replagal_in_USA_as_FDA_wants_more_trials.aspx.
- ↑ "Improvement in cardiac function in the cardiac variant of Fabry's disease with galactose-infusion therapy". The New England Journal of Medicine 345 (1): 25–32. July 2001. doi:10.1056/nejm200107053450104. PMID 11439944.
- ↑ 19.0 19.1 "Bacterial glycosidases for the production of universal red blood cells". Nature Biotechnology 25 (4): 454–64. April 2007. doi:10.1038/nbt1298. PMID 17401360.
- ↑ "In vitro inhibition and intracellular enhancement of lysosomal alpha-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives". European Journal of Biochemistry 267 (13): 4179–86. July 2000. doi:10.1046/j.1432-1327.2000.01457.x. PMID 10866822.
- ↑ "Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor". Nature Medicine 5 (1): 112–5. January 1999. doi:10.1038/4801. PMID 9883849.
- ↑ "Group B erythrocytes enzymatically converted to group O survive normally in A, B, and O individuals". Science 215 (4529): 168–70. January 1982. doi:10.1126/science.6274021. PMID 6274021. Bibcode: 1982Sci...215..168G.
- ↑ "Modifying the red cell surface: towards an ABO-universal blood supply". British Journal of Haematology 140 (1): 3–12. January 2008. doi:10.1111/j.1365-2141.2007.06839.x. PMID 17970801.
- ↑ "Biochemistry and Genetics of the ABO, Lewis, and P blood group systems". Advances in Human Genetics (Springer US) 10: 1–136, 379–85. 1980. doi:10.1007/978-1-4615-8288-5_1. ISBN 9781461582908. PMID 6156588.
- ↑ "Genetics of ABO, H, Lewis, X and related antigens". Vox Sanguinis 51 (3): 161–71. 1986. doi:10.1111/j.1423-0410.1986.tb01946.x. PMID 2433836.
- ↑ "ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution". Vox Sanguinis 56 (1): 1–20. 1989. doi:10.1159/000460912. PMID 2464874.
- ↑ "α-Galactosidase from Bacillus megaterium VHM1 and its application in removal of flatulence-causing factors from soymilk". Journal of Microbiology and Biotechnology 20 (11): 1546–54. November 2010. doi:10.4014/jmb.0912.12012. PMID 21124061.
Further reading
- "[Phylogenetic analysis of alpha-galactosidases of the GH27 family]" (in ru). Molekuliarnaia Biologiia 38 (3): 463–76. 2004. PMID 15285616. Republished as: "Phylogenetic Analysis of α-Galactosidases of the GH27 Family". Molecular Biology 38 (3): 388–400. 2004. doi:10.1023/B:MBIL.0000032210.97006.de.
- "Molecular basis of Fabry disease: mutations and polymorphisms in the human α-galactosidase A gene". Human Mutation 3 (2): 103–11. 1994. doi:10.1002/humu.1380030204. PMID 7911050.
- "[Gaucher's and Fabry's diseases: biochemical and genetic aspects]" (in fr). Journal de la Société de Biologie 196 (2): 135–40. 2002. doi:10.1051/jbio/2002196020135. INIST:13891620. PMID 12360742.
- "[Fabry's disease (alpha-galactosidase-A deficiency): physiopathology, clinical signs, and genetic aspects]" (in fr). Journal de la Société de Biologie 196 (2): 161–73. 2002. doi:10.1051/jbio/2002196020161. INIST:13891623. PMID 12360745.
- "Genotype and phenotype in Fabry disease: analysis of the Fabry Outcome Survey". Acta Paediatrica 94 (447): 87–92; discussion 79. March 2005. doi:10.1080/08035320510031045. PMID 15895718.
- "Fabry disease". Drugs of Today 42 (1): 65–70. January 2006. doi:10.1358/dot.2006.42.1.957357. PMID 16511611.
- "Clinical results of enzyme replacement therapy in Fabry disease: a comprehensive review of literature". International Journal of Clinical Practice 61 (2): 293–302. February 2007. doi:10.1111/j.1742-1241.2006.01237.x. PMID 17263716.
- "Studies on human liver alpha-galactosidases. I. Purification of alpha-galactosidase A and its enzymatic properties with glycolipid and oligosaccharide substrates". The Journal of Biological Chemistry 254 (20): 9994–10000. October 1979. doi:10.1016/S0021-9258(19)86663-2. PMID 39940. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=39940.
- "Point mutations in the upstream region of the alpha-galactosidase A gene exon 6 in an atypical variant of Fabry disease". Human Genetics 89 (1): 29–32. April 1992. doi:10.1007/BF00207037. PMID 1315715.
- "Overexpression of human alpha-galactosidase A results in its intracellular aggregation, crystallization in lysosomes, and selective secretion". The Journal of Cell Biology 119 (5): 1137–50. December 1992. doi:10.1083/jcb.119.5.1137. PMID 1332979.
- "An atypical variant of Fabry's disease with manifestations confined to the myocardium". The New England Journal of Medicine 324 (6): 395–9. February 1991. doi:10.1056/NEJM199102073240607. PMID 1846223.
- "A case of Fabry's disease in a patient with no α-galactosidase A activity caused by a single amino acid substitution of Pro-40 by Ser". FEBS Letters 259 (2): 353–6. January 1990. doi:10.1016/0014-5793(90)80046-L. PMID 2152885.
- "Alpha-galactosidase A gene rearrangements causing Fabry disease. Identification of short direct repeats at breakpoints in an Alu-rich gene". The Journal of Biological Chemistry 265 (16): 9319–26. June 1990. doi:10.1016/S0021-9258(19)38851-9. PMID 2160973. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=2160973.
- "Identification of point mutations in the α-galactosidase A gene in classical and atypical hemizygotes with Fabry disease". American Journal of Human Genetics 47 (5): 784–9. November 1990. PMID 2171331.
- "Fabry disease: six gene rearrangements and an exonic point mutation in the α-galactosidase gene". The Journal of Clinical Investigation 83 (4): 1390–9. April 1989. doi:10.1172/JCI114027. PMID 2539398.
- "Nucleotide sequence of the human α-galactosidase A gene". Nucleic Acids Research 17 (8): 3301–2. April 1989. doi:10.1093/nar/17.8.3301. PMID 2542896.
- "Structural organization of the human α-galactosidase A gene: further evidence for the absence of a 3′ untranslated region". Proceedings of the National Academy of Sciences of the United States of America 85 (11): 3903–7. June 1988. doi:10.1073/pnas.85.11.3903. PMID 2836863. Bibcode: 1988PNAS...85.3903B.
- "A genomic clone containing the promoter for the gene encoding the human lysosomal enzyme, α-galactosidase A". Gene 58 (2–3): 177–88. 1987. doi:10.1016/0378-1119(87)90374-X. PMID 2892762.
- "Human α-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme". Proceedings of the National Academy of Sciences of the United States of America 83 (13): 4859–63. July 1986. doi:10.1073/pnas.83.13.4859. PMID 3014515. Bibcode: 1986PNAS...83.4859B.
- "Synthesis and processing of α-galactosidase A in human fibroblasts. Evidence for different mutations in Fabry disease". The Journal of Biological Chemistry 262 (5): 2062–5. February 1987. doi:10.1016/S0021-9258(18)61618-7. PMID 3029062. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=3029062.
- "Signal sequence and DNA-mediated expression of human lysosomal α-galactosidase A". European Journal of Biochemistry 165 (2): 275–80. June 1987. doi:10.1111/j.1432-1033.1987.tb11438.x. PMID 3036505.
External links
- alpha-Galactosidase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human GLA genome location and GLA gene details page in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.