Biology:Exosome (vesicle)
| Exosome (extracellular vesicle) | |
|---|---|
 Exosome cross-section showing hsp70 protein | |
| Anatomical terminology |
Exosomes are membrane-bound extracellular vesicles (EVs) that are produced in the endosomal compartment of most eukaryotic cells.[1][2][3] In multicellular organisms, exosomes and other EVs are found in biological fluids including saliva, blood, urine and cerebrospinal fluid.[4] EVs have specialized functions in physiological processes, from coagulation and waste management to intercellular communication.[5]
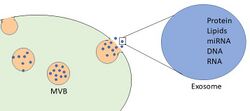
Exosomes are formed through the inward budding of a late endosome, also known as a multivesicular body (MVB).[6] The intraluminal vesicles (ILVs) of the multivesicular body (MVB) bud inward into the endosomal lumen. If the MVB fuses with the cell surface (the plasma membrane), these ILVs are released as exosomes.[7] Exosomes were also identified within the tissue matrix, coined Matrix-Bound Nanovesicles (MBV).[8] They are also released in vitro by cultured cells into their growth medium.[5][9][10]
Since the size of exosomes is limited by that of the parent MVB, exosomes are generally thought to be smaller than most other EVs, from about 30 to 150 nanometres (nm) in diameter: around the same size as many lipoproteins but much smaller than cells.[11][5] Compared with EVs in general, it is unclear whether exosomes have unique characteristics or functions or can be separated or distinguished effectively from other EVs.[1]
EVs in circulation carry genetic material and proteins from their cell of origin, proteo-transcriptomic signatures that act as biomarkers.[6][4][5][12] In the case of cancer cells, exosomes may show differences in size, shape, morphology, and canonical markers from their donor cells. They may encapsulate relevant information that can be used for disease detection.[4][6] Consequently, there is a growing interest in clinical applications of EVs as biomarkers and therapies alike,[13] prompting establishment of an International Society for Extracellular Vesicles (ISEV) and a scientific journal devoted to EVs, the Journal of Extracellular Vesicles.
Background
Exosomes were first discovered in the maturing mammalian reticulocyte (immature red blood cell) by Stahl and group in 1983 [14] and Johnstone and group in 1983[15] further termed 'exosomes' by Johnstone and group in 1987.[16] Exosomes were shown to participate in selective removal of many plasma membrane proteins[17] as the reticulocyte becomes a mature red blood cell (erythrocyte). In the reticulocyte, as in most mammalian cells, portions of the plasma membrane are regularly internalized as endosomes, with 50 to 180% of the plasma membrane being recycled every hour.[18] In turn, parts of the membranes of some endosomes are subsequently internalized as smaller vesicles. Such endosomes are called multivesicular bodies because of their appearance, with many small vesicles, (ILVs or "intralumenal endosomal vesicles"), inside the larger body. The ILVs become exosomes if the MVB merges with the cell membrane, releasing the internal vesicles into the extracellular space.[19]
Exosomes contain various molecular constituents of their cell of origin, including proteins and RNA. Although the exosomal protein composition varies with the cell and tissue of origin, most exosomes contain an evolutionarily-conserved common set of protein molecules. The protein content of a single exosome, given certain assumptions of protein size and configuration, and packing parameters, can be about 20,000 molecules.[20] The cargo of mRNA and miRNA in exosomes was first discovered at the University of Gothenburg in Sweden.[21]
The content of exosomes changes depending on the cells of origin, and they thereby reflect their originating cells. Analysis of the dynamic variation of exosomes may provide a valuable means of monitoring diseases.[22] In that study, the differences in cellular and exosomal mRNA and miRNA content was described, as well as the functionality of the exosomal mRNA cargo. Exosomes have also been shown to carry double-stranded DNA.[23]
Exosomes can transfer molecules from one cell to another via membrane vesicle trafficking, thereby influencing the immune system, such as dendritic cells and B cells, and may play a functional role in mediating adaptive immune responses to pathogens and tumors.[24][25] Therefore, scientists who are actively researching the role that exosomes may play in cell-to-cell signaling, often hypothesize that delivery of their cargo RNA molecules can explain biological effects. For example, mRNA in exosomes has been suggested to affect protein production in the recipient cell.[21][26][27] However, another study has suggested that miRNAs in exosomes secreted by mesenchymal stem cells (MSC) are predominantly pre- and not mature miRNAs.[28] Because the authors of this study did not find RNA-induced silencing complex-associated proteins in these exosomes, they suggested that only the pre-miRNAs, but not the mature miRNAs in MSC exosomes, have the potential to be biologically active in the recipient cells. Multiple mechanisms have been reported to be involved in loading miRNAs into exosomes, including specific motifs in the miRNA sequences, interactions with lncRNAs localized to the exosomes, interactions with RBPs, and post-translational modifications of Ago.[29]
Conversely, exosome production and content may be influenced by molecular signals received by the cell of origin. As evidence for this hypothesis, tumor cells exposed to hypoxia secrete exosomes with enhanced angiogenic and metastatic potential, suggesting that tumor cells adapt to a hypoxic microenvironment by secreting exosomes to stimulate angiogenesis or facilitate metastasis to more favorable environment.[30]
Terminology
Evolving consensus in the field is that the term "exosome" should be applied strictly to an EV of endosomal origin. Since it can be difficult to prove such an origin after an EV has left the cell, variations on the term "extracellular vesicle" are often appropriate instead.[1][31]
Research
Exosomes from red blood cells contain the transferrin receptor that is absent in mature erythrocytes. Dendritic cell-derived exosomes express MHC I, MHC II, and costimulatory molecules and have been proven to be able to induce and enhance antigen-specific T cell responses in vivo. In addition, the first exosome-based cancer vaccination platforms are being explored in early clinical trials.[32] Exosomes can also be released into urine by the kidneys, and their detection might serve as a diagnostic tool.[33][34][35] Urinary exosomes may be useful as treatment response markers in prostate cancer.[36][37] Exosomes secreted from tumour cells can deliver signals to surrounding cells and have been shown to regulate myofibroblast differentiation.[38] In melanoma, tumor-derived vesicles can enter lymphatics and interact with subcapsular sinus macrophages and B cells in lymph nodes.[39] A recent investigation showed that exosome release positively correlates with the invasiveness of ovarian cancer.[40] Exosomes released from tumors into the blood may also have diagnostic potential. Exosomes are remarkably stable in bodily fluids strengthening their utility as reservoirs for disease biomarkers.[41][42] Patient blood samples stored in biorepositories can be used for biomarker analysis as colorectal cancer cell-derived exosomes spiked into blood plasma could be recovered after 90 days of storage at various temperatures.[43]
In malignancies such as cancer, the regulatory circuit that guards exosome homeostasis is co-opted to promote cancer cell survival and metastasis.[44][27] In breast cancers, neratinib, a novel pan-ERBB inhibitor, is able to downmodulate the amount of HER2 released by exosomes, thus potentially reducing tumor dissemination.[45]
Urinary exosomes have also proven to be useful in the detection of many pathologies, such as genitourinary cancers and mineralocorticoid hypertension, through their protein and miRNA cargo."[46][13]
With neurodegenerative disorders, exosomes appear to play a role in the spread of alpha-synuclein, and are being actively investigated as a tool to both monitor disease progression as well as a potential vehicle for delivery of drug and stem cell based therapy.[47]
An online open access database containing genomic information for exosome content has been developed to catalyze research development within the field.[47]
Exosomes and intercellular communication
Scientists are actively researching the role that exosomes may play in cell-to-cell signaling, hypothesizing that because exosomes can merge with and release their contents into cells that are distant from their cell of origin (see membrane vesicle trafficking), they may influence processes in the recipient cell.[48] For example, RNA that is shuttled from one cell to another, known as "exosomal shuttle RNA," could potentially affect protein production in the recipient cell.[26][21] The role played by exosomes in cell-cell or interorgan communication and metabolic regulation was reviewed by Samuelson and Vidal-Puig in 2018.[49] By transferring molecules from one cell to another, exosomes from certain cells of the immune system, such as dendritic cells and B cells, may play a functional role in mediating adaptive immune responses to pathogens and tumors.[24][39] Exosomal export of miRNA molecules is also linked to the arrest of inter cellular miRNA levels and affect their functionality by arresting them on heavy polysomes.[50]
Conversely, exosome production and content may be influenced by molecular signals received by the cell of origin. As evidence for this hypothesis, tumor cells exposed to hypoxia secrete exosomes with enhanced angiogenic and metastatic potential, suggesting that tumor cells adapt to a hypoxic microenvironment by secreting exosomes to stimulate angiogenesis or facilitate metastasis to more favorable environment.[30] It has recently been shown that exosomal protein content may change during the progression of chronic lymphocytic leukemia.[51]
A study hypothesized that intercellular communication of tumor exosomes could mediate further regions of metastasis for cancer. Hypothetically, exosomes can plant tumor information, such as tainted RNA, into new cells to prepare for cancer to travel to that organ for metastasis. The study found that tumor exosomal communication has the ability to mediate metastasis to different organs. Furthermore, even when tumor cells have a disadvantage for replicating, the information planted at these new regions, organs, can aid in the expansion of organ specific metastasis.[52]
Exosomes carry cargo, which can augment innate immune responses. For example, exosomes derived from Salmonella enterica-infected macrophages but not exosomes from uninfected cells stimulate naive macrophages and dendritic cells to secrete pro-inflammatory cytokines such as TNF-α, RANTES, IL-1ra, MIP-2, CXCL1, MCP-1, sICAM-1, GM-CSF, and G-CSF. Proinflammatory effects of exosomes are partially attributed to lipopolysaccharide, which is encapsulated within exosomes.[53]
Exosomes also mediate the cross talk between the embryo and maternal compartment during implantation.They help to exchange ubiquitous protein, glycoproteins, DNA and mRNA.[54]
Exosome biogenesis, secretion, and uptake
Exosomes biogenesis
Exosome formation starts with the invagination of the multi-vesicular bodies (MVBs) or late endosomes to generate intraluminal vesicles (ILVs).[55] There are various proposed mechanisms for formation of MVBs, vesicle budding, and sorting. The most studied and well known is the endosomal sorting complex required for transport (ESCRT) dependent pathway. ESCRT machinery mediates the ubiquitinated pathway consisting of protein complexes; ESCRT-0, -I, -II, -III, and associated ATPase Vps4. ESCRT 0 recognizes and retains ubiquitinated proteins marked for packaging in the late endosomal membrane. ESCRT I/II recognizes the ESCRT 0 and starts creating involution of the membrane into the MVB. ESCRTIII forms a spiral-shaped structure constricting the neck. ATPase VPS4 protein drives the membrane scission.[56] Syndecan-syntenin-ALIX exosome biogenesis pathway are one of the ESCRT-independent or non-canonical pathways for exosome biogenesis.[57]
Exosome secretion
The MVBs once formed are trafficked to the internal side of the plasma membrane. These MVBs are transported to the plasma membrane leading to fusion.[55] Many studies have shown that MVBs having higher cholesterol content fuse with the plasma membrane thus releasing exosomes.[58] The Rab proteins especially Rab 7 attached to the MVB recognizes its effector receptor. The SNARE complex (soluble N- ethylmaleimide- sensitive fusion attachment protein receptor) from the MVB and the plasma membrane interacts and mediates fusion.
Exosome uptake
Specific targeting by exosomes is an active area of research. The exact mechanisms of exosome targeting is limited to a few general mechanisms like docking of the exosomes with specific proteins, sugars, and lipid, or micropinocytosis. The internalized exosomes are targeted to the endosomes which release their content in the recipient cell.[59][60]
Sorting and packaging of cargoes in exosomes
Exosomes contain different cargoes; proteins, lipids, and nucleic acids. These cargoes are specifically sorted and packaged into exosomes. The contents packaged into exosomes are cell type specific and also influenced by cellular conditions.[55] Exosomal microRNAs (exomiRs) and proteins are sorted and packaged in exosomes. Villarroya-Beltri and colleagues identified a conserved GGAG specific motif, EXOmotif, in the miRNA packaged in the exosomes which was absent in the cytosolic miRNA (CLmiRNA), which binds to sumoylated heterogeneous nuclear riboprotein (hnRNP) A2B1 for exosome specific miRNA packaging[61] Proteins are packaged in ESCRT, tertraspanins, lipid- dependent mechanisms.[62] Exosomes are enriched in cholesterol, sphingomyelin, saturated phosphatidylcholine and phosphatidylethanolamine as compared to the plasma membrane of the cell.[62]
Isolation
The isolation and detection of exosomes has proven to be complicated.[5][63] Due to the complexity of body fluids, physical separation of exosomes from cells and similar-sized particles is challenging. Isolation of exosomes using differential ultracentrifugation results in co-isolation of protein and other contaminants and incomplete separation of vesicles from lipoproteins.[64] Combining ultracentrifugation with micro-filtration or a gradient can improve purity.[65][66] Single step isolation of extracellular vesicles by size-exclusion chromatography has been demonstrated to provide greater efficiency for recovering intact vesicles over centrifugation,[67] although a size-based technique alone will not be able to distinguish exosomes from other vesicle types. To isolate a pure population of exosomes a combination of techniques is necessary, based on both physical (e.g. size, density) and biochemical parameters (e.g. presence/absence of certain proteins involved in their biogenesis).[64][68] The use of reference materials such as trackable recombinant EV will assist in mitigating technical variation introduced during sample preparation and analysis.[69][70] Novel selective isolation methodology has been using a combination of immunoaffinity chromatography and asymmetric-flow field-flow fractionation to reduce the contamination from lipoproteins and other proteins when isolating from blood plasma.[71][72]
Often, functional as well as antigenic assays are applied to derive useful information from multiple exosomes. Well-known examples of assays to detect proteins in total populations of exosomes are mass spectrometry and Western blot. However, a limitation of these methods is that contaminants may be present that affect the information obtained from such assays. Preferably, information is derived from single exosomes. Relevant properties of exosomes to detect include size, density, morphology, composition, and zeta potential.[73]
Detection
Since the diameter of exosomes is typically below 100 nm and because they have a low refractive index, exosomes are below the detection range of many currently used techniques. A number of miniaturized systems, exploiting nanotechnology and microfluidics, have been developed to expedite exosome analyses. These new systems include a microNMR device,[74] a nanoplasmonic chip,[75] and a magneto-electrochemical sensor[76] for protein profiling; and an integrated fluidic cartridge for RNA detection.[77] Flow cytometry is an optical method to detect exosomes in suspension. Nevertheless, the applicability of flow cytometry to detect single exosomes is still inadequate due to limited sensitivity and potential measurement artifacts such as swarm detection.[78] Other methods to detect single exosomes are atomic force microscopy,[79] nanoparticle tracking analysis,[80] Raman microspectroscopy,[81] tunable resistive pulse sensing, and transmission electron microscopy.[78][45]
Bioinformatics analysis
Exosomes contain RNA, proteins, lipids and metabolites that is reflective of the cell type of origin. As exosomes contain numerous proteins, RNA and lipids, large scale analysis including proteomics and transcriptomics is often performed. Currently, to analyse these data, non-commercial tools such as FunRich[82] can be used to identify over-represented groups of molecules. With the advent of Next generation sequencing technologies, the research on exosomes have been accelerated in not only cancer but various diseases. Recently, bioinformatics based analysis of RNA-Seq data of exosomes extracted from Trypanosoma cruzi has showed the association of these extracellular vesicles with various important gene products that strengthens the probability of finding biomarkers for Chagas disease.[83]
Therapeutics and carriers of drugs
Increasingly, exosomes are being recognized as potential therapeutics as they have the ability to elicit potent cellular responses in vitro and in vivo.[84][85][86] Exosomes mediate regenerative outcomes in injury and disease that recapitulate observed bioactivity of stem cell populations.[87][88] Mesenchymal stem cell exosomes were found to activate several signaling pathways important in wound healing (Akt, ERK, and STAT3), bone fracture repair [89][90] and participates in the regulation of immune-mediated responses[91][92] and inflammatory diseases.[93][94] They induce the expression of a number of growth factors (hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF1), nerve growth factor (NGF), and stromal-derived growth factor-1 (SDF1)).[95] Exosomes secreted by human circulating fibrocytes, a population of mesenchymal progenitors involved in normal wound healing via paracrine signaling, exhibited in-vitro proangiogenic properties, activated diabetic dermal fibroblasts, induced the migration and proliferation of diabetic keratinocytes, and accelerated wound closure in diabetic mice in vivo. Important components of the exosomal cargo were heat shock protein-90α, total and activated signal transducer and activator of transcription 3, proangiogenic (miR-126, miR-130a, miR-132) and anti-inflammatory (miR124a, miR-125b) microRNAs, and a microRNA regulating collagen deposition (miR-21).[96] Researchers have also found that exosomes released from oral keratinocytes can accelerate wound healing, even when human exosomes were applied to rat wounds.[97] Exosomes can be considered a promising carrier for effective delivery of small interfering RNA due to their existence in body's endogenous system and high tolerance.[98][99] Patient-derived exosomes have been employed as a novel cancer immunotherapy in several clinical trials.[100]
Exosome-mediated delivery of superoxide dismutase extends life-span in Caenorhabditis elegans, apparently by reducing the level of reactive oxygen species.[101] Thus this system is being studied for its anti-aging potential.[101] This delivery system also improved survival under conditions of oxidative stress and heat.[101]
Exosomes offer distinct advantages that uniquely position them as highly effective drug carriers.[102] Composed of cellular membranes with multiple adhesive proteins on their surface, exosomes are known to specialize in cell–cell communications and provide an exclusive approach for the delivery of various therapeutic agents to target cells.[103] For example, researchers used exosomes as a vehicle for the delivery of cancer drug paclitaxel. They placed the drug inside exosomes derived from white blood cells, which were then injected into mice with drug-resistant lung cancer. Importantly, incorporation of paclitaxel into exosomes increased cytotoxicity more than 50 times as a result of nearly complete co-localization of airway-delivered exosomes with lung cancer cells.[104]
Unapproved marketing
Different forms of unproven exosomes are being marketed in the U.S. for a wide variety of health conditions by clinic firms, without authorization from the FDA. Often, these firms also sell non-FDA-approved stem cell injections as well. In late 2019, the FDA issued an advisory warning about noncompliant marketing of exosomes and injuries to patients in Nebraska related to injections of exosomes.[105] The agency also indicated that exosomes are officially drug products requiring pre-market approval. In 2020, the FDA cautioned several firms about marketing or use of exosomes for COVID-19 and other health conditions.[106][107][108]
See also
- Prostasomes
- Microvesicles
- Vesicles
- ExoCarta – database of molecules shown to be present in exosomes[109]
References
- ↑ 1.0 1.1 1.2 "Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines". Journal of Extracellular Vesicles 7 (1): 1535750. 2018. doi:10.1080/20013078.2018.1535750. PMID 30637094.
- ↑ "Biological properties of extracellular vesicles and their physiological functions". Journal of Extracellular Vesicles 4: 27066. 2015. doi:10.3402/jev.v4.27066. PMID 25979354.
- ↑ "Shedding light on the cell biology of extracellular vesicles". Nature Reviews. Molecular Cell Biology 19 (4): 213–228. April 2018. doi:10.1038/nrm.2017.125. PMID 29339798. https://hal.archives-ouvertes.fr/hal-02359760/file/NRMCB%20MAA.pdf.
- ↑ 4.0 4.1 4.2 Nonaka, T; Wong, DTW (13 June 2022). "Saliva Diagnostics.". Annual Review of Analytical Chemistry 15 (1): 107–121. doi:10.1146/annurev-anchem-061020-123959. PMID 35696523. Bibcode: 2022ARAC...15..107N.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Classification, functions, and clinical relevance of extracellular vesicles". Pharmacological Reviews 64 (3): 676–705. July 2012. doi:10.1124/pr.112.005983. PMID 22722893.
- ↑ 6.0 6.1 6.2 Chen, TY; Gonzalez-Kozlova, E; Soleymani, T; La Salvia, S; Kyprianou, N; Sahoo, S; Tewari, AK; Cordon-Cardo, C et al. (17 June 2022). "Extracellular vesicles carry distinct proteo-transcriptomic signatures that are different from their cancer cell of origin.". iScience 25 (6): 104414. doi:10.1016/j.isci.2022.104414. ISSN 2589-0042. PMID 35663013. Bibcode: 2022iSci...25j4414C.
- ↑ Janas, AM; Sapoń, K; Janas, T; Stowell, MH; Janas, T (June 2016). "Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases.". Biochimica et Biophysica Acta (BBA) - Biomembranes 1858 (6): 1139–51. doi:10.1016/j.bbamem.2016.02.011. PMID 26874206.
- ↑ "Matrix-bound nanovesicles within ECM bioscaffolds". Science Advances 2 (6): e1600502. June 2016. doi:10.1126/sciadv.1600502. PMID 27386584. Bibcode: 2016SciA....2E0502H.
- ↑ "Exosomes: from biogenesis and secretion to biological function". Immunology Letters 107 (2): 102–8. November 2006. doi:10.1016/j.imlet.2006.09.005. PMID 17067686.
- ↑ "Exosomes populate the cerebrospinal fluid of preterm infants with post-haemorrhagic hydrocephalus". International Journal of Developmental Neuroscience 73: 59–65. April 2019. doi:10.1016/j.ijdevneu.2019.01.004. PMID 30639393. http://orca.cf.ac.uk/118564/1/CLAYTON%2C%20Aled%20-%20Exosomes%20populate%20the%20cerebrospinal%20fluid%20of%20preterm.pdf.
- ↑ Rudraprasad, Dhanwini; Rawat, Aadish; Joseph, Joveeta (2022-01-01). "Exosomes, extracellular vesicles and the eye" (in en). Experimental Eye Research 214: 108892. doi:10.1016/j.exer.2021.108892. ISSN 0014-4835. PMID 34896308. https://www.sciencedirect.com/science/article/pii/S0014483521004589.
- ↑ Loewy, MA (14 March 2023). "Saliva: The next frontier in cancer detection" (in en). Knowable Magazine | Annual Reviews. doi:10.1146/knowable-031323-1. https://knowablemagazine.org/article/health-disease/2023/saliva-next-frontier-cancer-detection.
- ↑ 13.0 13.1 "Urinary extracellular vesicle biomarkers in urological cancers: From discovery towards clinical implementation". The International Journal of Biochemistry & Cell Biology 99: 236–256. June 2018. doi:10.1016/j.biocel.2018.04.009. PMID 29654900. https://figshare.com/articles/journal_contribution/7067903.
- ↑ "Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing". Biochemical and Biophysical Research Communications 113 (2): 650–8. June 1983. doi:10.1016/0006-291X(83)91776-X. PMID 6870878.
- ↑ "Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor". Cell 33 (3): 967–78. July 1983. doi:10.1016/0092-8674(83)90040-5. PMID 6307529.
- ↑ "Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes)". The Journal of Biological Chemistry 262 (19): 9412–20. July 1987. doi:10.1016/S0021-9258(18)48095-7. PMID 3597417.
- ↑ "Exosomes: a common pathway for a specialized function". Journal of Biochemistry 140 (1): 13–21. July 2006. doi:10.1093/jb/mvj128. PMID 16877764.
- ↑ "Endosome maturation". The EMBO Journal 30 (17): 3481–500. August 2011. doi:10.1038/emboj.2011.286. PMID 21878991.
- ↑ "Mechanisms of pathogen entry through the endosomal compartments". Nature Reviews. Molecular Cell Biology 7 (7): 495–504. July 2006. doi:10.1038/nrm1959. PMID 16773132.
- ↑ Maguire, Greg (2016) Exosomes: smart nanospheres for drug delivery naturally produced by stem cells. In: Fabrication and Self Assembly of Nanobiomaterials. Elsevier pp. 179-209.
- ↑ 21.0 21.1 21.2 "Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells". Nature Cell Biology 9 (6): 654–9. June 2007. doi:10.1038/ncb1596. PMID 17486113.
- ↑ "Tumour-derived exosomes as a signature of pancreatic cancer - liquid biopsies as indicators of tumour progression". Oncotarget 8 (10): 17279–17291. March 2017. doi:10.18632/oncotarget.13973. PMID 27999198.
- ↑ "Double-stranded DNA in exosomes: a novel biomarker in cancer detection". Cell Research 24 (6): 766–9. June 2014. doi:10.1038/cr.2014.44. PMID 24710597.
- ↑ 24.0 24.1 "Role of exosomes in immune regulation". Journal of Cellular and Molecular Medicine 10 (2): 364–75. 2006. doi:10.1111/j.1582-4934.2006.tb00405.x. PMID 16796805.
- ↑ "Exosomes in immunoregulation of chronic lung diseases". Allergy 72 (4): 534–544. April 2017. doi:10.1111/all.13086. PMID 27859351.
- ↑ 26.0 26.1 "Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences". Nature Communications 2 (2): 180. February 2011. doi:10.1038/ncomms1180. PMID 21285958. Bibcode: 2011NatCo...2..180B.
- ↑ 27.0 27.1 "Glioblastoma multiforme-derived extracellular vesicles drive normal astrocytes towards a tumour-enhancing phenotype". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 373 (1737): 20160477. January 2018. doi:10.1098/rstb.2016.0477. PMID 29158308.
- ↑ "Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs". Nucleic Acids Research 38 (1): 215–24. January 2010. doi:10.1093/nar/gkp857. PMID 19850715.
- ↑ "Regulation of microRNA function in animals". Nature Reviews. Molecular Cell Biology 20 (1): 21–37. January 2019. doi:10.1038/s41580-018-0045-7. PMID 30108335.
- ↑ 30.0 30.1 "Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes". Molecular & Cellular Proteomics 9 (6): 1085–99. June 2010. doi:10.1074/mcp.M900381-MCP200. PMID 20124223.
- ↑ "Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature". Journal of Extracellular Vesicles 8 (1): 1648167. 2019. doi:10.1080/20013078.2019.1648167. PMID 31489144.
- ↑ "Prospects for exosomes in immunotherapy of cancer". Journal of Cellular and Molecular Medicine 10 (2): 376–88. 2006. doi:10.1111/j.1582-4934.2006.tb00406.x. PMID 16796806.
- ↑ "Identification and proteomic profiling of exosomes in human urine". Proceedings of the National Academy of Sciences of the United States of America 101 (36): 13368–73. September 2004. doi:10.1073/pnas.0403453101. PMID 15326289. Bibcode: 2004PNAS..10113368P.
- ↑ "Urinary Exosome Protein Database". NHLBI. 2009-05-12. http://dir.nhlbi.nih.gov/papers/lkem/exosome/.
- ↑ "Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer". British Journal of Cancer 100 (10): 1603–7. May 2009. doi:10.1038/sj.bjc.6605058. PMID 19401683.
- ↑ "Fat capsules carry markers for deadly prostate cancer". The Medical News. 2009-05-13. http://www.news-medical.net/news/2009/05/12/Fat-capsules-carry-markers-for-deadly-prostate-cancer.aspx.
- ↑ "Can urinary exosomes act as treatment response markers in prostate cancer?". Journal of Translational Medicine 7 (1): 4. January 2009. doi:10.1186/1479-5876-7-4. PMID 19138409.
- ↑ "Cancer exosomes trigger fibroblast to myofibroblast differentiation". Cancer Research 70 (23): 9621–30. December 2010. doi:10.1158/0008-5472.CAN-10-1722. PMID 21098712.
- ↑ 39.0 39.1 "SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions". Science 352 (6282): 242–6. April 2016. doi:10.1126/science.aaf1328. PMID 26989197. Bibcode: 2016Sci...352..242P.
- ↑ "Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200". Journal of Translational Medicine 12: 4. January 2014. doi:10.1186/1479-5876-12-4. PMID 24393345.
- ↑ "Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives". Journal of Extracellular Vesicles 7 (1): 1442985. 2018. doi:10.1080/20013078.2018.1442985. PMID 29535851.
- ↑ "Mass spectrometry for glycan biomarker discovery". TrAC Trends in Analytical Chemistry 100: 7–14. 2018. doi:10.1016/j.trac.2017.12.015.
- ↑ "Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma". Proteomics 13 (22): 3354–64. November 2013. doi:10.1002/pmic.201300282. PMID 24115447.
- ↑ "Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance". Trends in Pharmacological Sciences 37 (7): 606–617. July 2016. doi:10.1016/j.tips.2016.04.006. PMID 27157716.
- ↑ 45.0 45.1 "Imaging of Endocytic Trafficking and Extracellular Vesicles Released Under Neratinib Treatment in ERBB2+ Breast Cancer Cells". The Journal of Histochemistry and Cytochemistry 69 (7): 461–473. July 2021. doi:10.1369/00221554211026297. PMID 34126793.
- ↑ "Urinary Exosomes and Their Cargo: Potential Biomarkers for Mineralocorticoid Arterial Hypertension?". Frontiers in Endocrinology 8: 230. 2017-09-08. doi:10.3389/fendo.2017.00230. PMID 28951728.
- ↑ 47.0 47.1 "A Critical Assessment of Exosomes in the Pathogenesis and Stratification of Parkinson's Disease". Journal of Parkinson's Disease 7 (4): 569–576. 2017. doi:10.3233/JPD-171176. PMID 28922170.
- ↑ "Function of extracellular vesicle-associated miRNAs in metastasis". Cell and Tissue Research 365 (3): 621–41. September 2016. doi:10.1007/s00441-016-2430-x. PMID 27289232. https://figshare.com/articles/journal_contribution/7067858.
- ↑ "Fed-EXosome: extracellular vesicles and cell-cell communication in metabolic regulation". Essays in Biochemistry 62 (2): 165–175. May 2018. doi:10.1042/EBC20170087. PMID 29717059.
- ↑ "Polysome arrest restricts miRNA turnover by preventing exosomal export of miRNA in growth-retarded mammalian cells". Molecular Biology of the Cell 26 (6): 1072–83. March 2015. doi:10.1091/mbc.E14-11-1521. PMID 25609084.
- ↑ "S100-A9 protein in exosomes from chronic lymphocytic leukemia cells promotes NF-κB activity during disease progression". Blood 130 (6): 777–788. August 2017. doi:10.1182/blood-2017-02-769851. PMID 28596424.
- ↑ "Tumour exosome integrins determine organotropic metastasis". Nature 527 (7578): 329–35. November 2015. doi:10.1038/nature15756. PMID 26524530. Bibcode: 2015Natur.527..329H.
- ↑ "Salmonella enterica Serovar Typhimurium Alters the Extracellular Proteome of Macrophages and Leads to the Production of Proinflammatory Exosomes". Infection and Immunity 86 (2): e00386–17. February 2018. doi:10.1128/IAI.00386-17. PMID 29158431.
- ↑ Kurian, Noble K.; Modi, Deepak (2019). "Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy". Journal of Assisted Reproduction and Genetics 36 (2): 189–198. doi:10.1007/s10815-018-1343-x. PMID 30362057.
- ↑ 55.0 55.1 55.2 "Current knowledge on exosome biogenesis and release". Cellular and Molecular Life Sciences 75 (2): 193–208. January 2018. doi:10.1007/s00018-017-2595-9. PMID 28733901.
- ↑ "Molecular mechanism of multivesicular body biogenesis by ESCRT complexes". Nature 464 (7290): 864–9. April 2010. doi:10.1038/nature08849. PMID 20305637. Bibcode: 2010Natur.464..864W.
- ↑ "Syndecan-syntenin-ALIX regulates the biogenesis of exosomes". Nature Cell Biology 14 (7): 677–85. June 2012. doi:10.1038/ncb2502. PMID 22660413.
- ↑ "Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O". The Journal of Histochemistry and Cytochemistry 50 (1): 43–55. January 2002. doi:10.1177/002215540205000105. PMID 11748293.
- ↑ "Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication". Nature Cell Biology 21 (1): 9–17. January 2019. doi:10.1038/s41556-018-0250-9. PMID 30602770.
- ↑ "The importance of cellular and exosomal miRNAs in mesenchymal stem cell osteoblastic differentiation". Scientific Reports 11 (1): 5953. March 2021. doi:10.1038/s41598-021-85306-2. PMID 33723364. Bibcode: 2021NatSR..11.5953S.
- ↑ "Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs". Nature Communications 4 (1): 2980. December 2013. doi:10.1038/ncomms3980. PMID 24356509. Bibcode: 2013NatCo...4.2980V.
- ↑ 62.0 62.1 "Sorting it out: regulation of exosome loading". Seminars in Cancer Biology 28: 3–13. October 2014. doi:10.1016/j.semcancer.2014.04.009. PMID 24769058.
- ↑ "Exosomal miRNAs as cancer biomarkers and therapeutic targets". Journal of Extracellular Vesicles 5: 31292. 2016. doi:10.3402/jev.v5.31292. PMID 27440105.
- ↑ 64.0 64.1 "Modern isolation and separation techniques for extracellular vesicles". Journal of Chromatography A 1636: 461773. January 2021. doi:10.1016/j.chroma.2020.461773. PMID 33316564.
- ↑ "Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes". Methods 56 (2): 293–304. February 2012. doi:10.1016/j.ymeth.2012.01.002. PMID 22285593.
- ↑ "The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling". Journal of Extracellular Vesicles 3: 24858. 2014. doi:10.3402/jev.v3.24858. PMID 25317274.
- ↑ "Single-step isolation of extracellular vesicles by size-exclusion chromatography". Journal of Extracellular Vesicles 3: 23430. 2014. doi:10.3402/jev.v3.23430. PMID 25279113.
- ↑ "Preparation of Multi-omics Grade Extracellular Vesicles by Density-Based Fractionation of Urine". STAR Protocols 1 (2): 100073. September 2020. doi:10.1016/j.xpro.2020.100073. PMID 33111109.
- ↑ "Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine". Journal of Extracellular Vesicles 9 (1): 1736935. 11 March 2020. doi:10.1080/20013078.2020.1736935. PMID 32284825.
- ↑ "The generation and use of recombinant extracellular vesicles as biological reference material". Nature Communications 10 (1): 3288. July 2019. doi:10.1038/s41467-019-11182-0. PMID 31337761. Bibcode: 2019NatCo..10.3288G.
- ↑ "Fast isolation of highly specific population of platelet-derived extracellular vesicles from blood plasma by affinity monolithic column, immobilized with anti-human CD61 antibody". Analytica Chimica Acta 1091: 160–168. December 2019. doi:10.1016/j.aca.2019.09.022. PMID 31679569. Bibcode: 2019AcAC.1091..160M.
- ↑ "Automated On-Line Isolation and Fractionation System for Nanosized Biomacromolecules from Human Plasma". Analytical Chemistry 92 (19): 13058–13065. October 2020. doi:10.1021/acs.analchem.0c01986. PMID 32893620.
- ↑ "Optical and non-optical methods for detection and characterization of microparticles and exosomes". Journal of Thrombosis and Haemostasis 8 (12): 2596–607. December 2010. doi:10.1111/j.1538-7836.2010.04074.x. PMID 20880256.
- ↑ "Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy". Nature Medicine 18 (12): 1835–40. December 2012. doi:10.1038/nm.2994. PMID 23142818.
- ↑ "Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor". Nature Biotechnology 32 (5): 490–5. May 2014. doi:10.1038/nbt.2886. PMID 24752081.
- ↑ "Integrated Magneto-Electrochemical Sensor for Exosome Analysis". ACS Nano 10 (2): 1802–9. February 2016. doi:10.1021/acsnano.5b07584. PMID 26808216.
- ↑ "Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma". Nature Communications 6: 6999. May 2015. doi:10.1038/ncomms7999. PMID 25959588. Bibcode: 2015NatCo...6.6999S.
- ↑ 78.0 78.1 "Single vs. swarm detection of microparticles and exosomes by flow cytometry". Journal of Thrombosis and Haemostasis 10 (5): 919–30. May 2012. doi:10.1111/j.1538-7836.2012.04683.x. PMID 22394434.
- ↑ "Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles". Journal of Thrombosis and Haemostasis 8 (2): 315–23. February 2010. doi:10.1111/j.1538-7836.2009.03654.x. PMID 19840362.
- ↑ "Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis". Nanomedicine 7 (6): 780–8. December 2011. doi:10.1016/j.nano.2011.04.003. PMID 21601655.
- ↑ "Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy". Journal of Extracellular Vesicles 1: 19179. 2012. doi:10.3402/jev.v1i0.19179. PMID 24009887.
- ↑ "FunRich: An open access standalone functional enrichment and interaction network analysis tool". Proteomics 15 (15): 2597–601. August 2015. doi:10.1002/pmic.201400515. PMID 25921073.
- ↑ "Mining SNPs in extracellular vesicular transcriptome of Trypanosoma cruzi: a step closer to early diagnosis of neglected Chagas disease". PeerJ 4: e2693. 2016. doi:10.7717/peerj.2693. PMID 27904804.
- ↑ "Exosomes and Their Therapeutic Potentials of Stem Cells". Stem Cells International 2016: 7653489. 2016. doi:10.1155/2016/7653489. PMID 26770213.
- ↑ "Exosomes and their Therapeutic Applications.". Advances In Pharmaceutical Cell Therapy: Principles Of Cell-based Biopharmaceuticals. Singapore: World Scientific. 2016. pp. 477–501. ISBN 978-981-4616-80-5. https://books.google.com/books?id=JFsGCwAAQBAJ&pg=PA477.
- ↑ "Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis". Stem Cells International 2016: 5029619. 2016. doi:10.1155/2016/5029619. PMID 27994623.
- ↑ "Preclinical translation of exosomes derived from mesenchymal stem/stromal cells". Stem Cells 38 (1): 15–21. January 2020. doi:10.1002/stem.3061. PMID 31381842.
- ↑ "Exosomes for repair, regeneration and rejuvenation". Expert Opinion on Biological Therapy 16 (4): 489–506. 2016. doi:10.1517/14712598.2016.1131976. PMID 26817494.
- ↑ "MSC-derived Exosomes Promote Bone Fracture Repair". Stem Cells Portal. 2 January 2017. http://stemcellsportal.com/article-scans/msc-derived-exosomes-promote-bone-fracture-repair.
- ↑ "Extracellular Vesicles: Immunomodulatory messengers in the context of tissue repair/regeneration". European Journal of Pharmaceutical Sciences 98: 86–95. February 2017. doi:10.1016/j.ejps.2016.09.017. PMID 27644894.
- ↑ "Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells". Frontiers in Immunology 5: 556. 2014. doi:10.3389/fimmu.2014.00556. PMID 25414703.
- ↑ "The immunomodulatory activity of extracellular vesicles derived from endometrial mesenchymal stem cells on CD4+ T cells is partially mediated by TGFbeta". Journal of Tissue Engineering and Regenerative Medicine 12 (10): 2088–2098. October 2018. doi:10.1002/term.2743. PMID 30058282.
- ↑ "Fibrin glue mesh fixation combined with mesenchymal stem cells or exosomes modulates the inflammatory reaction in a murine model of incisional hernia". Acta Biomaterialia 71: 318–329. April 2018. doi:10.1016/j.actbio.2018.02.014. PMID 29462710.
- ↑ "Mesenchymal Stem Cell-Derived Exosomes: Immunomodulatory Evaluation in an Antigen-Induced Synovitis Porcine Model". Frontiers in Veterinary Science 4: 39. 2017. doi:10.3389/fvets.2017.00039. PMID 28377922.
- ↑ "Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro". Stem Cells and Development 24 (14): 1635–47. July 2015. doi:10.1089/scd.2014.0316. PMID 25867197.
- ↑ "Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice". Biochemical and Biophysical Research Communications 467 (2): 303–9. November 2015. doi:10.1016/j.bbrc.2015.09.166. PMID 26454169.
- ↑ "Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing". Journal of Extracellular Vesicles 8 (1): 1565264. 20 January 2019. doi:10.1080/20013078.2019.1565264. PMID 30719240.
- ↑ "Delivery of Small Interfering RNAs to Cells via Exosomes". SiRNA Delivery Methods. Methods in Molecular Biology. 1364. 2016. pp. 105–25. doi:10.1007/978-1-4939-3112-5_10. ISBN 978-1-4939-3111-8.
- ↑ "Exosomes: Natural Carriers for siRNA Delivery". Current Pharmaceutical Design 21 (31): 4556–65. 2015. doi:10.2174/138161282131151013190112. PMID 26486142.
- ↑ "Designer exosomes as next-generation cancer immunotherapy". Nanomedicine 12 (1): 163–9. January 2016. doi:10.1016/j.nano.2015.09.011. PMID 26500074.
- ↑ 101.0 101.1 101.2 Shao, X.; Zhang, M.; Chen, Y.; Sun, S.; Yang, S.; Li, Q. (2023). "Exosome-mediated delivery of superoxide dismutase for anti-aging studies in Caenorhabditis elegans". International Journal of Pharmaceutics 641: 123090. doi:10.1016/j.ijpharm.2023.123090. PMID 37268030.
- ↑ Askenase, Philip W., Artificial nanoparticles are not as good as the real thing, Outlook, Nature, June 17, 2020
- ↑ "Using exosomes, naturally-equipped nanocarriers, for drug delivery". Journal of Controlled Release 219: 396–405. December 2015. doi:10.1016/j.jconrel.2015.07.030. PMID 26241750.
- ↑ "Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells". Nanomedicine 12 (3): 655–664. April 2016. doi:10.1016/j.nano.2015.10.012. PMID 26586551.
- ↑ Center for Biologics Evaluation and Research (2019-12-20). "Public Safety Notification on Exosome Products" (in en). FDA. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/public-safety-notification-exosome-products.
- ↑ "Kimera Labs FDA letter cites exosomes for COVID-19, more issues" (in en-US). 2020-04-23. https://ipscell.com/2020/04/kimera-labs-fda-letter-exosomes-for-covid-19-more-issues/.
- ↑ "Limited Reporting of Adverse Events Tied to Regenerative Treatments Leaves Consumers Vulnerable". 31 July 2020. https://pew.org/3jP1Gui.
- ↑ "FDA Warning Letters Week of 4/20/2020: PMA, IDE, & Untitled Letter to Stem Cell Firm" (in en-US). 2020-04-27. https://redica.com/pharma-medical-devices-fda-warning-letters-week-of-4-20-2020-device-untitled-letters/.
- ↑ "ExoCarta: A compendium of exosomal proteins and RNA". Proteomics 9 (21): 4997–5000. November 2009. doi:10.1002/pmic.200900351. PMID 19810033.
Further reading
- Ibrahim, H. M.; Mohammed-Geba, K.; Tawfic, A. A.; & El-Magd, M. A. (2019). Camel milk exosomes modulate cyclophosphamide-induced oxidative stress and immuno-toxicity in rats. Food and Function, 10(11), 7523–7532.
 |