Biology:Alpha-synuclein
 Generic protein structure example |
Alpha-synuclein (aSyn) is a protein that, in humans, is encoded by the SNCA gene.[1] Alpha-synuclein is a neuronal protein that regulates synaptic vesicle trafficking and subsequent neurotransmitter release.[2][3]
It is abundant in the brain, while smaller amounts are found in the heart, muscle and other tissues. In the brain, alpha-synuclein is found mainly in the axon terminals of presynaptic neurons.[1] Within these terminals, alpha-synuclein interacts with phospholipids[4] and proteins.[1][5][6] Presynaptic terminals release chemical messengers, called neurotransmitters, from compartments known as synaptic vesicles. The release of neurotransmitters relays signals between neurons and is critical for normal brain function.[1]
In Parkinson's disease and other synucleinopathies, insoluble forms of alpha-synuclein accumulate as inclusions in Lewy bodies.[7]
Familial Parkinson's disease is associated with mutations in the -synuclein (SNCA) gene. In the process of seeded nucleation, alpha-synuclein acquires a cross-sheet structure similar to other amyloids.[8]
The human alpha-synuclein protein is made of 140 amino acids.[9][10][11] An alpha-synuclein fragment, known as the non-amyloid beta (non-abeta) component (NAC) of Alzheimer's disease amyloid, originally found in an amyloid-enriched fraction, was shown to be a fragment of its precursor protein, NACP.[9] It was later determined that NACP was the human homologue of Torpedo synuclein. Therefore, NACP is now referred to as human alpha-synuclein.[12]
Tissue expression
Alpha-synuclein is a synuclein protein primarily found in neural tissue, making up as much as one percent of all proteins in the cytosol of brain cells.[13] It is expressed highly in neurons within the frontal cortex, hippocampus, striatum, and olfactory bulb,[13] but can also be found in the non-neuronal glial cells.[14] In melanocytes, SNCA protein expression may be regulated by microphthalmia-associated transcription factor (MITF).[15]
It has been established that alpha-synuclein is extensively localized in the nucleus of mammalian brain neurons, suggesting a role of alpha-synuclein in the nucleus.[16] Synuclein is however found predominantly in the presynaptic termini, in both free or membrane-bound forms,[17] with roughly 15% of synuclein being membrane-bound at any moment in neurons.[18]
It has also been shown that alpha-synuclein is localized in neuronal mitochondria.[19][20] Alpha-synuclein is highly expressed in the mitochondria in olfactory bulb, hippocampus, striatum and thalamus, where the cytosolic alpha-synuclein is also rich. However, the cerebral cortex and cerebellum are two exceptions, which contain rich cytosolic alpha-synuclein but very low levels of mitochondrial alpha-synuclein. It has been shown that alpha-synuclein is localized in the inner membrane of mitochondria, and that the inhibitory effect of alpha-synuclein on complex I activity of the mitochondrial respiratory chain is dose-dependent. Thus, it is suggested that alpha-synuclein in mitochondria is differentially expressed in different brain regions and the background levels of mitochondrial alpha-synuclein may be a potential factor affecting mitochondrial function and predisposing some neurons to degeneration.[20]
At least three isoforms of synuclein are produced through alternative splicing.[21] The majority form of the protein, and the one most investigated, is the full-length protein of 140 amino acids. Other isoforms are alpha-synuclein-126, which lacks residues 41-54 due to loss of exon 3; and alpha-synuclein-112,[22] which lacks residues 103-130 due to loss of exon 5.[21]
In the enteric nervous system (ENS)
First characterisations of aSyn aggregates in the ENS of PD patients has been performed on autopsied specimens in the late 1980s.[23] It is yet unknown if the microbiome changes associated with PD are consequential to the illness process or main pathophysiology, or both.[24]
Individuals diagnosed with various synucleinopathies often display constipation and other GI dysfunctions years prior to the onset of movement dysfunction. [25]
Alpha synuclein potentially connects the gut-brain axis in Parkinson's disease patients. Common inherited Parkinson disease is associated with mutations in the alpha-synuclein (SNCA) gene. In the process of seeded nucleation, alpha-synuclein acquires a cross-sheet structure similar to other amyloids. [23]
The Enterobacteriaceae, which are quite common in the human gut, can create curli, which are functional amyloid proteins. The unfolded amyloid CsgA, which is secreted by bacteria and later aggregates extracellularly to create biofilms, mediates adherence to epithelial cells, and aids in bacteriophage defense, forms the curli fibers. Oral injection of curli-producing bacteria can also boost formation and aggregation of the amyloid protein Syn in old rats and nematodes. Host inflammation responses in the intestinal tract and periphery are modulated by curli exposure. Studies in biochemistry show that endogenous, bacterial chaperones of curli are capable of briefly interacting with Syn and controlling its aggregation.[25]
The clinical and pathological findings support the hypothesis that aSyn disease in PD occurs via a gut-brain pathway. For early diagnosis and early management in the phase of creation and propagation of aSyn, it is therefore of utmost importance to identify pathogenic aSyn in the digestive system, for example, by gastrointestinal tract (GIT) biopsies.[23]
According to a growing body of research, intestinal dysbiosis may be a major factor in the development of Parkinson's disease by encouraging intestinal permeability, gastrointestinal inflammation, and the aggregation and spread of asyn.[23]
Not just the CNS but other peripheral tissues, such as the GIT, have physiological aSyn expression as well as its phosphorylated variants.[26] As suggested by Borghammer and Van Den Berge (2019), one approach is to recognise the possibility of PD subtypes with various aSyn propagation methods, including either a peripheral nervous system (PNS)-first or a CNS-first route.[27]
While the GI tract has been linked to other neurological disorders such autism spectrum disorder, depression, anxiety, and Alzheimer's disease, protein aggregation and/or inflammation in the gut represent a new topic of investigation in synucleinopathies.[25]
Structure
Alpha-synuclein in solution is considered to be an intrinsically disordered protein, i.e. it lacks a single stable 3D structure.[28][29] As of 2014, an increasing number of reports suggest, however, the presence of partial structures or mostly structured oligomeric states in the solution structure of alpha-synuclein even in the absence of lipids. This trend is also supported by a large number of single molecule (optical tweezers) measurements on single copies of monomeric alpha-synuclein as well as covalently enforced dimers or tetramers of alpha-synuclein.[30]
Alpha-synuclein is specifically upregulated in a discrete population of presynaptic terminals of the brain during a period of acquisition-related synaptic rearrangement.[31] It has been shown that alpha-synuclein significantly interacts with tubulin,[32] and that alpha-synuclein may have activity as a potential microtubule-associated protein, like tau.[33] Evidence suggests that alpha-synuclein functions as a molecular chaperone in the formation of SNARE complexes.[34][35] In particular, it simultaneously binds to phospholipids of the plasma membrane via its N-terminus domain and to synaptobrevin-2 via its C-terminus domain, with increased importance during synaptic activity.[36] Indeed, there is growing evidence that alpha-synuclein is involved in the functioning of the neuronal Golgi apparatus and vesicle trafficking.[37]
Apparently, alpha-synuclein is essential for normal development of the cognitive functions. Knock-out mice with the targeted inactivation of the expression of alpha-synuclein show impaired spatial learning and working memory.[38]
Interaction with lipid membranes
Experimental evidence has been collected on the interaction of alpha-synuclein with membrane and its involvement with membrane composition and turnover. Yeast genome screening has found that several genes that deal with lipid metabolism and mitochondrial fusion play a role in alpha-synuclein toxicity.[39][40] Conversely, alpha-synuclein expression levels can affect the viscosity and the relative amount of fatty acids in the lipid bilayer.[41]
Alpha-synuclein is known to directly bind to lipid membranes, associating with the negatively charged surfaces of phospholipids.[41] Alpha-synuclein forms an extended helical structure on small unilamellar vesicles.[42] A preferential binding to small vesicles has been found.[43] The binding of alpha-synuclein to lipid membranes has complex effects on the latter, altering the bilayer structure and leading to the formation of small vesicles.[44] Alpha-synuclein has been shown to bend membranes of negatively charged phospholipid vesicles and form tubules from large lipid vesicles.[45] Using cryo-EM it was shown that these are micellar tubes of ~5-6 nm diameter.[46] Alpha-synuclein has also been shown to form lipid disc-like particles similar to apolipoproteins.[47] EPR studies have shown that the structure of alpha synuclein is dependent on the binding surface.[48] The protein adopts a broken-helical conformation on lipoprotein particles while it forms an extended helical structure on lipid vesicles and membrane tubes.[48] Studies have also suggested a possible antioxidant activity of alpha-synuclein in the membrane.[49]
Membrane interaction of alpha-synuclein modulates or affects its rate of aggregation.[50] The membrane-mediated modulation of aggregation is very similar to that observed for other amyloid proteins such as IAPP and abeta.[50] Aggregated states of alpha-synuclein permeate the membrane of lipid vesicles.[51] They are formed upon interaction with peroxidation-prone polyunsaturated fatty acids (PUFA) but not with monounsaturated fatty acids[52] and the binding of lipid autoxidation-promoting transition metals such as iron or copper provokes oligomerization of alpha-synuclein.[53] The aggregated alpha-synuclein has a specific activity for peroxidized lipids and induces lipid autoxidation in PUFA-rich membranes of both neurons and astrocytes, decreasing resistance to apoptosis.[54] Lipid autoxidation is inhibited if the cells are pre-incubated with isotope-reinforced PUFAs (D-PUFA).[55]
Function
Although the function of alpha-synuclein is not well understood, studies suggest that it plays a role in restricting the mobility of synaptic vesicles, consequently attenuating synaptic vesicle recycling and neurotransmitter release.[56][57][58][59][60][61][5] An alternate view is that alpha-synuclein binds to VAMP2 (a synaptobrevin) and stabilizes SNARE complexes;[36][62][63][64][65] though recent studies indicate that alpha-synuclein–VAMP2 binding is critical for alpha-synuclein-mediated attenuation of synaptic vesicle recycling, connecting the two seemingly divergent views.[5] It may also help regulate the release of dopamine, a type of neurotransmitter that is critical for controlling the start and stop of voluntary and involuntary movements.[1]
Alpha-synuclein modulates DNA repair processes, including repair of double-strand breaks (DSBs).[66] DNA damage response markers co-localize with alpha-synuclein to form discrete foci in human cells and mouse brain. Depletion of alpha-synuclein in human cells causes increased introduction of DNA DSBs after exposure to bleomycin and reduced ability to repair these DSBs. In addition, alpha-synuclein knockout mice display a higher level of DSBs, and this problem can be alleviated by transgenic reintroduction of human alpha-synuclein. Alpha-synuclein promotes the DSB repair pathway referred to as non-homologous end joining.[66] The DNA repair function of alpha-synuclein appears to be compromised in Lewy body inclusion bearing neurons, and this may trigger cell death.
Proneurogenic function of alpha-synuclein
In some neurodegenerative diseases, alpha-synuclein produces insoluble inclusion bodies. These diseases, known as synucleinopathies, are connected with either higher levels of normal alpha-synuclein or its mutant variants.[67] The normal physiological role of Snca, however, has not yet been thoroughly explained. In fact, physiological Snca has been demonstrated to have a neuroprotective impact by inhibiting apoptosis induced by several types of apoptotic stimuli, or by regulating the expression of proteins involved in apoptotic pathways. Recently it has been demonstrated that up-regulation of alpha-synuclein in the dentate gyrus (a neurogenic niche where new neurons are generated throughout life) activates stem cells, in a model of premature neural aging. This model shows reduced expression of alpha-synuclein and reduced proliferation of stem cells, as is physiologically observed during aging. Exogenous alpha-synuclein in the dentate gyrus is able to rescue this defect. Moreover, alpha-synuclein also boosts the proliferation of dentate gyrus progenitor neural cells in wild-type young mice. Thus, alpha-synuclein represents an effector for neural stem and progenitor cell activation.[68] Similarly, alpha-synuclein has been found to be required to maintain stem cells of the SVZ (subventricular zone, i.e., another neurogenic niche) in a cycling state.[69]
Sequence
Alpha-synuclein primary structure is usually divided in three distinct domains:
- Residues 1-60: An amphipathic N-terminal region dominated by four 11-residue repeats including the consensus sequence KTKEGV. This sequence has a structural alpha helix propensity similar to apolipoproteins-binding domains.[70] It is a highly conserved terminal that interacts with acidic lipid membranes, and all the discovered point mutations of the SNCA gene are located within this terminal.[71]
- Residues 61-95: A central hydrophobic region which includes the non-amyloid-β component (NAC) region, involved in protein aggregation.[9] This domain is unique to alpha-synuclein among the synuclein family.[72]
- Residues 96-140: a highly acidic and proline-rich region which has no distinct structural propensity. This domain plays an important role in the function, solubility and interaction of alpha-synuclein with other proteins.[73][36]
Autoproteolytic activity
The use of high-resolution ion-mobility mass spectrometry (IMS-MS) on HPLC-purified alpha-synuclein in vitro has shown alpha-synuclein to be autoproteolytic (self-proteolytic), generating a variety of small molecular weight fragments upon incubation.[74] The 14.46 kDa protein was found to generate numerous smaller fragments, including 12.16 kDa (amino acids 14–133) and 10.44 kDa (40-140) fragments formed through C- and N-terminal truncation and a 7.27 kDa C-terminal fragment (72-140). The 7.27 kDa fragment, which contains the majority of the NAC region, aggregated considerably faster than full-length alpha-synuclein. It is possible that these autoproteolytic products play a role as intermediates or cofactors in the aggregation of alpha-synuclein in vivo.
Clinical significance
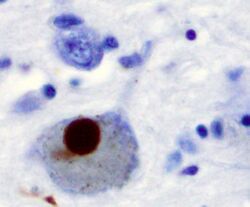
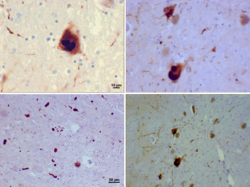
Alpha synuclein, having no single, well-defined tertiary structure, is an intrinsically disordered protein,[75][76] with a pI value of 4.7,[77] which, under certain pathological conditions, can misfold in a way that exposes its core hydrophobic residues to the intracellular milieu, thus providing the opportunity for hydrophobic interactions to occur with a similar, equally exposed protein.[76] This could lead to self assembly and subsequent aggregation into large, insoluble fibrils known as amyloids.[76] The conversion of soluble alpha synuclein into highly ordered, cross-β sheet, fibrillar structures does not, as previously thought, follow a two-step mechanism, rather, occurs through a series of transient, soluble oligomeric intermediates.[78][79] In 2011, two groups published their findings that unmutated α-synuclein forms a stably folded tetramer that resists aggregation, asserting that this folded tetramer represented the relevant in vivo structure in cells,[80][81] thereby relieving alpha synuclein of its disordered status. Proponents of the tetramer hypothesis argued that in vivo cross-linking in bacteria, primary neurons and human erythroleukemia cells confirmed the presence of labile, tetrameric species.[82][83][84] However, despite numerous in-cell NMR reports demonstrating that alpha synuclein is indeed monomeric and disordered in intact E. coli cells,[85][86][87][88][89][90][91][92] it is still a matter of debate in the field despite an ever growing mountain of conflicting reports.[89][93][94] Nevertheless, alpha-synuclein aggregates to form insoluble fibrils in pathological conditions characterized by Lewy bodies, such as Parkinson's disease, dementia with Lewy bodies and multiple system atrophy.[95][96] These disorders are known as synucleinopathies. In vitro models of synucleinopathies revealed that aggregation of alpha-synuclein may lead to various cellular disorders including microtubule impairment, synaptic and mitochondrial dysfunctions, oxidative stress as well as dysregulation of Calcium signaling, proteasomal and lysosomal pathway.[97] Alpha-synuclein is the primary structural component of Lewy body fibrils. Occasionally, Lewy bodies contain tau protein;[98] however, alpha-synuclein and tau constitute two distinctive subsets of filaments in the same inclusion bodies.[99] Alpha-synuclein pathology is also found in both sporadic and familial cases with Alzheimer's disease.[100]
The aggregation mechanism of alpha-synuclein is uncertain. There is evidence of a structured intermediate rich in beta structure that can be the precursor of aggregation and, ultimately, Lewy bodies.[101] A single molecule study in 2008 suggests alpha-synuclein exists as a mix of unstructured, alpha-helix, and beta-sheet-rich conformers in equilibrium. Mutations or buffer conditions known to improve aggregation strongly increase the population of the beta conformer, thus suggesting this could be a conformation related to pathogenic aggregation.[102] One theory is that the majority of alpha-synuclein aggregates are located in the presynapse as smaller deposits which causes synaptic dysfunction.[103] Among the strategies for treating synucleinopathies are compounds that inhibit aggregation of alpha-synuclein. It has been shown that the small molecule cuminaldehyde inhibits fibrillation of alpha-synuclein.[104] The Epstein-Barr virus has been implicated in these disorders.[105]
In rare cases of familial forms of Parkinson's disease, there is a mutation in the gene coding for alpha-synuclein. Five point mutations have been identified thus far: A53T,[106] A30P,[107] E46K,[108] H50Q,[109] and G51D;[110] however, in total, nineteen mutations in the SNCA gene have been associated with parkinsonism: A18T, A29S, A53E, A53V, E57A, V15A, T72M, L8I, V15D, M127I, P117S, M5T, G93A, E83Q, and A30G.[111]
It has been reported that some mutations influence the initiation and amplification steps of the aggregation process.[112][113] Genomic duplication and triplication of the gene appear to be a rare cause of Parkinson's disease in other lineages, although more common than point mutations.[114][115] Hence certain mutations of alpha-synuclein may cause it to form amyloid-like fibrils that contribute to Parkinson's disease. Over-expression of human wild-type or A53T-mutant alpha-synuclein in primates drives deposition of alpha-synuclein in the ventral midbrain, degeneration of the dopaminergic system and impaired motor performance.[116]
Certain sections of the alpha-synuclein protein may play a role in the tauopathies.[117][118][119]
A prion form of the protein alpha-synuclein may be a causal agent for the disease multiple system atrophy.[120][121][122]
Self-replicating "prion-like" amyloid assemblies of alpha-synuclein have been described that are invisible to the amyloid dye Thioflavin T and that can acutely spread in neurons in vitro and in vivo.[124]
This section needs additional citations for verification. (November 2015) (Learn how and when to remove this template message) |
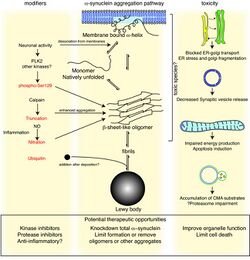
Antibodies against alpha-synuclein have replaced antibodies against ubiquitin as the gold standard for immunostaining of Lewy bodies.[125] The central panel in the figure to the right shows the major pathway for protein aggregation. Monomeric α-synuclein is natively unfolded in solution but can also bind to membranes in an α-helical form. It seems likely that these two species exist in equilibrium within the cell, although this is unproven. From in vitro work, it is clear that unfolded monomer can aggregate first into small oligomeric species that can be stabilized by β-sheet-like interactions and then into higher molecular weight insoluble fibrils. In a cellular context, there is some evidence that the presence of lipids can promote oligomer formation: α-synuclein can also form annular, pore-like structures that interact with membranes. The deposition of α-synuclein into pathological structures such as Lewy bodies is probably a late event that occurs in some neurons. On the left hand side are some of the known modifiers of this process. Electrical activity in neurons changes the association of α-synuclein with vesicles and may also stimulate polo-like kinase 2 (PLK2), which has been shown to phosphorylate α-synuclein at Ser129. Other kinases have also been proposed to be involved. As well as phosphorylation, truncation through proteases such as calpains, and nitration, probably through nitric oxide (NO) or other reactive nitrogen species that are present during inflammation, all modify synuclein such that it has a higher tendency to aggregate. The addition of ubiquitin (shown as a black spot) to Lewy bodies is probably a secondary process to deposition. On the right are some of the proposed cellular targets for α-synuclein mediated toxicity, which include (from top to bottom) ER-golgi transport, synaptic vesicles, mitochondria and lysosomes and other proteolytic machinery. In each of these cases, it is proposed that α-synuclein has detrimental effects, listed below each arrow, although at this time it is not clear if any of these are either necessary or sufficient for toxicity in neurons.
Protein-protein interactions
Alpha-synuclein has been shown to interact with
- Dopamine transporter,[126][127]
- Parkin (ligase),[128][129]
- Phospholipase D1,[130]
- SNCAIP,[131][132][133][134]
- Tau protein.[135][136][137]
- Beta amyloid[138]
See also
- Synuclein
- Contursi Terme - the village in Italy where a mutation in the α-synuclein gene led to a family history of Parkinson's disease
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Genetics Home Reference: SNCA". U.S. National Library of Medicine. 12 Nov 2013. http://ghr.nlm.nih.gov/gene/SNCA.
- ↑ "Snaring the function of alpha-synuclein". Cell 123 (3): 359–361. November 2005. doi:10.1016/j.cell.2005.10.017. PMID 16269324.
- ↑ "Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration". Cell 123 (3): 383–396. November 2005. doi:10.1016/j.cell.2005.09.028. PMID 16269331.
- ↑ "A broken alpha -helix in folded alpha -Synuclein". The Journal of Biological Chemistry 278 (17): 15313–15318. April 2003. doi:10.1074/jbc.M213128200. PMID 12586824.
- ↑ 5.0 5.1 5.2 "Functional cooperation of α-synuclein and VAMP2 in synaptic vesicle recycling". Proceedings of the National Academy of Sciences of the United States of America 116 (23): 11113–11115. June 2019. doi:10.1073/pnas.1903049116. PMID 31110017. Bibcode: 2019PNAS..11611113S.
- ↑ "Synapsins regulate α-synuclein functions". Proceedings of the National Academy of Sciences of the United States of America 116 (23): 11116–11118. June 2019. doi:10.1073/pnas.1903054116. PMID 31110014. Bibcode: 2019PNAS..11611116A.
- ↑ Spillantini, Maria Grazia; Schmidt, Marie Luise; Lee, Virginia M.-Y; Trojanowkski, John Q.; Jakes, Ross; Goedert, Michel (28 August 1997). "α-Synuclein in Lewy bodies". Nature 388 (6645): 839–840. doi:10.1038/42166. PMID 9278044. Bibcode: 1997Natur.388..839G.
- ↑ Neurobiology of brain disorders : biological basis of neurological and psychiatric disorders. London. 2015. ISBN 978-0-12-398280-3. OCLC 896232309. https://www.worldcat.org/oclc/896232309.
- ↑ 9.0 9.1 9.2 "Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease". Proceedings of the National Academy of Sciences of the United States of America 90 (23): 11282–11286. December 1993. doi:10.1073/pnas.90.23.11282. PMID 8248242. Bibcode: 1993PNAS...9011282U.
- ↑ "Characterization of the human alpha-synuclein gene: Genomic structure, transcription start site, promoter region and polymorphisms". Journal of Alzheimer's Disease 3 (5): 485–494. October 2001. doi:10.3233/JAD-2001-3508. PMID 12214035. http://iospress.metapress.com/content/jpmvj4dubjpm3b73/. Retrieved 2009-02-19.
- ↑ "Characterization of the human alpha-synuclein gene: Genomic structure, transcription start site, promoter region and polymorphisms: Erratum p489 Fig 3". J. Alzheimers Dis. 4 (4): 337. 2002. http://iospress.metapress.com/content/jpmvj4dubjpm3b73/. Retrieved 2009-02-19.
- ↑ "Identification of two distinct synucleins from human brain". FEBS Letters 345 (1): 27–32. May 1994. doi:10.1016/0014-5793(94)00395-5. PMID 8194594.
- ↑ 13.0 13.1 "The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system". Neuron 14 (2): 467–475. February 1995. doi:10.1016/0896-6273(95)90302-X. PMID 7857654.
- ↑ "α-Synuclein and Glia in Parkinson's Disease: A Beneficial or a Detrimental Duet for the Endo-Lysosomal System?". Cellular and Molecular Neurobiology 39 (2): 161–168. March 2019. doi:10.1007/s10571-019-00649-9. PMID 30637614.
- ↑ "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell & Melanoma Research 21 (6): 665–676. December 2008. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971.
- ↑ "Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody". Neuroscience 145 (2): 539–555. March 2007. doi:10.1016/j.neuroscience.2006.12.028. PMID 17275196.
- ↑ "Membrane association and protein conformation of alpha-synuclein in intact neurons. Effect of Parkinson's disease-linked mutations". The Journal of Biological Chemistry 275 (12): 8812–8816. March 2000. doi:10.1074/jbc.275.12.8812. PMID 10722726.
- ↑ "Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form". The Journal of Biological Chemistry 277 (1): 671–678. January 2002. doi:10.1074/jbc.M107045200. PMID 11679584.
- ↑ "Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody". Brain Research 1244: 40–52. December 2008. doi:10.1016/j.brainres.2008.08.067. PMID 18817762.
- ↑ 20.0 20.1 "alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity". Neuroscience Letters 454 (3): 187–192. May 2009. doi:10.1016/j.neulet.2009.02.056. PMID 19429081.
- ↑ 21.0 21.1 "Alpha-synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers". Acta Neuropathologica 112 (3): 237–251. September 2006. doi:10.1007/s00401-006-0104-6. PMID 16845533.
- ↑ "Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A beta component of Alzheimer's disease amyloid". Biochemical and Biophysical Research Communications 205 (2): 1366–1372. December 1994. doi:10.1006/bbrc.1994.2816. PMID 7802671.
- ↑ 23.0 23.1 23.2 23.3 "Alpha Synuclein Connects the Gut-Brain Axis in Parkinson's Disease Patients - A View on Clinical Aspects, Cellular Pathology and Analytical Methodology". Frontiers in Cell and Developmental Biology 8: 573696. 2020. doi:10.3389/fcell.2020.573696. PMID 33015066.
- ↑ "Digesting recent findings: gut alpha-synuclein, microbiome changes in Parkinson's disease" (in English). Trends in Endocrinology and Metabolism 33 (2): 147–157. February 2022. doi:10.1016/j.tem.2021.11.005. PMID 34949514.
- ↑ 25.0 25.1 25.2 "A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice". eLife 9: e53111. February 2020. doi:10.7554/eLife.53111. PMID 32043464.
- ↑ "Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson's disease". Acta Neuropathologica Communications 5 (1): 1. January 2017. doi:10.1186/s40478-016-0408-2. PMID 28057070.
- ↑ "Brain-First versus Gut-First Parkinson's Disease: A Hypothesis". Journal of Parkinson's Disease 9 (s2): S281–S295. 2019-10-30. doi:10.3233/jpd-191721. PMID 31498132.
- ↑ "Tryptophan fluorescence reveals structural features of alpha-synuclein oligomers". Journal of Molecular Biology 394 (5): 826–833. December 2009. doi:10.1016/j.jmb.2009.10.021. PMID 19837084. https://ris.utwente.nl/ws/files/6758594/Rooijen09tryptophan.pdf.
- ↑ "NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded". Biochemistry 35 (43): 13709–13715. October 1996. doi:10.1021/bi961799n. PMID 8901511.
- ↑ "Diverse metastable structures formed by small oligomers of α-synuclein probed by force spectroscopy". PLOS ONE 9 (1): e86495. January 2014. doi:10.1371/journal.pone.0086495. PMID 24475132. Bibcode: 2014PLoSO...986495N.
- ↑ "Characterization of a novel protein regulated during the critical period for song learning in the zebra finch". Neuron 15 (2): 361–372. August 1995. doi:10.1016/0896-6273(95)90040-3. PMID 7646890.
- ↑ "Tubulin seeds alpha-synuclein fibril formation". The Journal of Biological Chemistry 277 (3): 2112–2117. January 2002. doi:10.1074/jbc.M102981200. PMID 11698390.
- ↑ "Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein". Journal of Alzheimer's Disease 6 (4): 435–42; discussion 443–9. August 2004. doi:10.3233/JAD-2004-6412. PMID 15345814.
- ↑ "Snaring the function of alpha-synuclein". Cell 123 (3): 359–361. November 2005. doi:10.1016/j.cell.2005.10.017. PMID 16269324.
- ↑ "Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration". Cell 123 (3): 383–396. November 2005. doi:10.1016/j.cell.2005.09.028. PMID 16269331.
- ↑ 36.0 36.1 36.2 "Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro". Science 329 (5999): 1663–1667. September 2010. doi:10.1126/science.1195227. PMID 20798282. Bibcode: 2010Sci...329.1663B.
- ↑ "Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models". Science 313 (5785): 324–328. July 2006. doi:10.1126/science.1129462. PMID 16794039. Bibcode: 2006Sci...313..324C.
- ↑ "α-Synuclein knockout mice have cognitive impairments". Behavioural Brain Research 231 (1): 226–230. May 2012. doi:10.1016/j.bbr.2012.03.026. PMID 22469626.
- ↑ "Alpha-Synuclein Toxicity is Caused by Mitochondrial Dysfunction". Electronic Thesis and Dissertation Repository. 4 February 2019. https://ir.lib.uwo.ca/etd/6019.
- ↑ "Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein". Science 302 (5651): 1769–1772. December 2003. doi:10.1126/science.1090389. PMID 14657499. Bibcode: 2003Sci...302.1769W.
- ↑ 41.0 41.1 "Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation". Journal of Neurochemistry 103 (1): 17–37. October 2007. doi:10.1111/j.1471-4159.2007.04764.x. PMID 17623039.
- ↑ "Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement". Proceedings of the National Academy of Sciences of the United States of America 105 (50): 19666–19671. December 2008. doi:10.1073/pnas.0807826105. PMID 19066219. Bibcode: 2008PNAS..10519666J.
- ↑ "The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation". The Journal of Biological Chemistry 278 (41): 40186–40197. October 2003. doi:10.1074/jbc.M305326200. PMID 12885775.
- ↑ "A study of the regional effects of alpha-synuclein on the organization and stability of phospholipid bilayers". Biochemistry 45 (18): 5783–5792. May 2006. doi:10.1021/bi052151q. PMID 16669622.
- ↑ "Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins". The Journal of Biological Chemistry 285 (42): 32486–32493. October 2010. doi:10.1074/jbc.M110.139576. PMID 20693280.
- ↑ "Remodeling of lipid vesicles into cylindrical micelles by α-synuclein in an extended α-helical conformation". The Journal of Biological Chemistry 287 (35): 29301–29311. August 2012. doi:10.1074/jbc.M112.365817. PMID 22767608.
- ↑ "α-Synuclein oligomers with broken helical conformation form lipoprotein nanoparticles". The Journal of Biological Chemistry 288 (24): 17620–17630. June 2013. doi:10.1074/jbc.M113.476697. PMID 23609437.
- ↑ 48.0 48.1 "Membrane remodeling by amyloidogenic and non-amyloidogenic proteins studied by EPR". Journal of Magnetic Resonance 280: 127–139. July 2017. doi:10.1016/j.jmr.2017.02.014. PMID 28579098. Bibcode: 2017JMagR.280..127V.
- ↑ "Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles". Biochemistry 45 (26): 8135–8142. July 2006. doi:10.1021/bi052584t. PMID 16800638.
- ↑ 50.0 50.1 "Membranes as modulators of amyloid protein misfolding and target of toxicity". Biochimica et Biophysica Acta (BBA) - Biomembranes 1860 (9): 1863–1875. September 2018. doi:10.1016/j.bbamem.2018.04.011. PMID 29702073.
- ↑ "Ultrasensitive Measurement of Ca2+ Influx into Lipid Vesicles Induced by Protein Aggregates". Angewandte Chemie 56 (27): 7750–7754. June 2017. doi:10.1002/anie.201700966. PMID 28474754.
- ↑ "The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson's disease". Neuron 37 (4): 583–595. February 2003. doi:10.1016/s0896-6273(03)00024-2. PMID 12597857.
- ↑ "Inhibitors of alpha-synuclein oligomerization and toxicity: a future therapeutic strategy for Parkinson's disease and related disorders". Experimental Brain Research 173 (2): 223–233. August 2006. doi:10.1007/s00221-006-0539-y. PMID 16733698.
- ↑ "Alpha-synuclein, lipids and Parkinson's disease". Progress in Lipid Research 49 (4): 420–428. October 2010. doi:10.1016/j.plipres.2010.05.004. PMID 20580911.
- ↑ "Lipid peroxidation is essential for α-synuclein-induced cell death". Journal of Neurochemistry 133 (4): 582–589. May 2015. doi:10.1111/jnc.13024. PMID 25580849.
- ↑ "Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis". The Journal of Neuroscience 26 (46): 11915–11922. November 2006. doi:10.1523/JNEUROSCI.3821-06.2006. PMID 17108165.
- ↑ "Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis". Neuron 65 (1): 66–79. January 2010. doi:10.1016/j.neuron.2009.12.023. PMID 20152114.
- ↑ "A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration". The Journal of Neuroscience 30 (24): 8083–8095. June 2010. doi:10.1523/JNEUROSCI.1091-10.2010. PMID 20554859.
- ↑ "α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis". The Journal of Neuroscience 32 (30): 10129–10135. July 2012. doi:10.1523/JNEUROSCI.0535-12.2012. PMID 22836248.
- ↑ "Synucleins regulate the kinetics of synaptic vesicle endocytosis". The Journal of Neuroscience 34 (28): 9364–9376. July 2014. doi:10.1523/JNEUROSCI.4787-13.2014. PMID 25009269.
- ↑ "α-synuclein multimers cluster synaptic vesicles and attenuate recycling". Current Biology 24 (19): 2319–2326. October 2014. doi:10.1016/j.cub.2014.08.027. PMID 25264250.
- ↑ "Cell Biology and Pathophysiology of α-Synuclein". Cold Spring Harbor Perspectives in Medicine 8 (3): a024091. March 2018. doi:10.1101/cshperspect.a024091. PMID 28108534.
- ↑ "Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities". The Journal of Neuroscience 32 (43): 15227–15242. October 2012. doi:10.1523/JNEUROSCI.3545-12.2012. PMID 23100443.
- ↑ "α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation". Proceedings of the National Academy of Sciences of the United States of America 111 (40): E4274–E4283. October 2014. doi:10.1073/pnas.1416598111. PMID 25246573. Bibcode: 2014PNAS..111E4274B.
- ↑ "Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2". eLife 2: e00592. April 2013. doi:10.7554/eLife.00592. PMID 23638301.
- ↑ 66.0 66.1 "Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders". Scientific Reports 9 (1): 10919. July 2019. doi:10.1038/s41598-019-47227-z. PMID 31358782. Bibcode: 2019NatSR...910919S.
- ↑ "A Focus on the Beneficial Effects of Alpha Synuclein and a Re-Appraisal of Synucleinopathies". Current Protein & Peptide Science 19 (6): 598–611. 2018. doi:10.2174/1389203718666171117110028. PMID 29150919.
- ↑ "Transcriptome Analysis in a Mouse Model of Premature Aging of Dentate Gyrus: Rescue of Alpha-Synuclein Deficit by Virus-Driven Expression or by Running Restores the Defective Neurogenesis". Frontiers in Cell and Developmental Biology 9: 696684. 2021. doi:10.3389/fcell.2021.696684. PMID 34485283.
- ↑ "Synaptic Regulator α-Synuclein in Dopaminergic Fibers Is Essentially Required for the Maintenance of Subependymal Neural Stem Cells". The Journal of Neuroscience 38 (4): 814–825. January 2018. doi:10.1523/JNEUROSCI.2276-17.2017. PMID 29217686.
- ↑ "The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease". Trends in Neurosciences 21 (6): 249–254. June 1998. doi:10.1016/S0166-2236(97)01213-7. PMID 9641537.
- ↑ "A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins". Journal of Molecular Biology 329 (4): 763–778. June 2003. doi:10.1016/S0022-2836(03)00520-5. PMID 12787676.
- ↑ "Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies". Acta Neuropathologica 131 (1): 49–73. January 2016. doi:10.1007/s00401-015-1485-1. PMID 26446103.
- ↑ "Carboxy-terminal truncations of mouse α-synuclein alter aggregation and prion-like seeding". FEBS Letters 594 (8): 1271–1283. April 2020. doi:10.1002/1873-3468.13728. PMID 31912891.
- ↑ "Autoproteolytic fragments are intermediates in the oligomerization/aggregation of the Parkinson's disease protein alpha-synuclein as revealed by ion mobility mass spectrometry". ChemBioChem 12 (18): 2740–2744. December 2011. doi:10.1002/cbic.201100569. PMID 22162214.
- ↑ "Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade". Annual Review of Biochemistry 86: 27–68. June 2017. doi:10.1146/annurev-biochem-061516-045115. PMID 28498720.
- ↑ 76.0 76.1 76.2 "Half a century of amyloids: past, present and future". Chemical Society Reviews 49 (15): 5473–5509. August 2020. doi:10.1039/c9cs00199a. PMID 32632432.
- ↑ "Isoelectric point-amyloid formation of α-synuclein extends the generality of the solubility and supersaturation-limited mechanism". Current Research in Structural Biology 2: 35–44. 2020. doi:10.1016/j.crstbi.2020.03.001. PMID 34235468.
- ↑ "Physicochemical properties of cells and their effects on intrinsically disordered proteins (IDPs)". Chemical Reviews 114 (13): 6661–6714. July 2014. doi:10.1021/cr400695p. PMID 24901537.
- ↑ "The amyloid state of proteins in human diseases". Cell 148 (6): 1188–1203. March 2012. doi:10.1016/j.cell.2012.02.022. PMID 22424229.
- ↑ "α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation". Nature 477 (7362): 107–110. August 2011. doi:10.1038/nature10324. PMID 21841800. Bibcode: 2011Natur.477..107B.
- ↑ "A soluble α-synuclein construct forms a dynamic tetramer". Proceedings of the National Academy of Sciences of the United States of America 108 (43): 17797–17802. October 2011. doi:10.1073/pnas.1113260108. PMID 22006323. Bibcode: 2011PNAS..10817797W.
- ↑ "In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells". The Journal of Biological Chemistry 288 (9): 6371–6385. March 2013. doi:10.1074/jbc.M112.403311. PMID 23319586.
- ↑ "Monomeric synucleins generate membrane curvature". The Journal of Biological Chemistry 288 (3): 1829–1840. January 2013. doi:10.1074/jbc.M112.418871. PMID 23184946.
- ↑ "N-Terminal acetylation is critical for forming α-helical oligomer of α-synuclein". Protein Science 21 (5): 601–605. May 2012. doi:10.1002/pro.2056. PMID 22407793.
- ↑ "Bacterial in-cell NMR of human α-synuclein: a disordered monomer by nature?". Biochemical Society Transactions 40 (5): 950–954. October 2012. doi:10.1042/BST20120096. PMID 22988846.
- ↑ "High-resolution characterization of intrinsic disorder in proteins: expanding the suite of (13)C-detected NMR spectroscopy experiments to determine key observables". ChemBioChem 12 (15): 2347–2352. October 2011. doi:10.1002/cbic.201100406. PMID 23106082.
- ↑ "In-cell NMR characterization of the secondary structure populations of a disordered conformation of α-synuclein within E. coli cells". PLOS ONE 8 (8): e72286. 26 August 2013. doi:10.1371/journal.pone.0072286. PMID 23991082. Bibcode: 2013PLoSO...872286W.
- ↑ "Protein dynamics in living cells studied by in-cell NMR spectroscopy". FEBS Letters 587 (8): 1008–1011. April 2013. doi:10.1016/j.febslet.2012.12.023. PMID 23318712.
- ↑ 89.0 89.1 "α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer". The Journal of Biological Chemistry 287 (19): 15345–15364. May 2012. doi:10.1074/jbc.M111.318949. PMID 22315227.
- ↑ "Molecular Crowding Stabilizes Both the Intrinsically Disordered Calcium-Free State and the Folded Calcium-Bound State of an RTX Protein: Implication for Toxin Secretion". Biophysical Journal 106 (2): 271a. January 2014. doi:10.1016/j.bpj.2013.11.1589. Bibcode: 2014BpJ...106R.271S.
- ↑ "Rapid distinction of intracellular and extracellular proteins using NMR diffusion measurements". Journal of the American Chemical Society 134 (28): 11312–11315. July 2012. doi:10.1021/ja304912c. PMID 22694283. https://discovery.ucl.ac.uk/id/eprint/1365837/.
- ↑ "Hydrogen exchange of monomeric alpha-synuclein shows unfolded structure persists at physiological temperature and is independent of molecular crowding in Escherichia coli". Protein Science 17 (8): 1434–1445. August 2008. doi:10.1110/ps.033803.107. PMID 18493022.
- ↑ "Properties of native brain α-synuclein". Nature 498 (7453): E4–6; discussion E6–7. June 2013. doi:10.1038/nature12125. PMID 23765500. Bibcode: 2013Natur.498E...4B.
- ↑ "Structural disorder of monomeric α-synuclein persists in mammalian cells". Nature 530 (7588): 45–50. February 2016. doi:10.1038/nature16531. PMID 26808899. Bibcode: 2016Natur.530...45T. https://repository.publisso.de/resource/frl:6410667.
- ↑ "Alpha-synuclein in Lewy bodies". Nature 388 (6645): 839–840. August 1997. doi:10.1038/42166. PMID 9278044. Bibcode: 1997Natur.388..839G.
- ↑ "Alpha synuclein in neurodegenerative disorders: murderer or accomplice?". Nature Medicine 4 (7): 755–757. July 1998. doi:10.1038/nm0798-755. PMID 9662355. https://zenodo.org/record/1233447.
- ↑ "In vitro models of synucleinopathies: informing on molecular mechanisms and protective strategies". Journal of Neurochemistry 150 (5): 535–565. September 2019. doi:10.1111/jnc.14707. PMID 31004503.
- ↑ "Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in lewy bodies in sporadic Parkinson's disease and in dementia with Lewy bodies". Brain Research 843 (1–2): 53–61. October 1999. doi:10.1016/S0006-8993(99)01848-X. PMID 10528110.
- ↑ "NACP/alpha-synuclein and tau constitute two distinctive subsets of filaments in the same neuronal inclusions in brains from a family of parkinsonism and dementia with Lewy bodies: double-immunolabeling fluorescence and electron microscopic studies". Acta Neuropathologica 100 (2): 115–121. August 2000. doi:10.1007/s004010050002. PMID 10963357.
- ↑ "NACP/alpha-synuclein, NAC, and beta-amyloid pathology of familial Alzheimer's disease with the E184D presenilin-1 mutation: a clinicopathological study of two autopsy cases". Acta Neuropathologica 104 (6): 637–648. December 2002. doi:10.1007/s00401-002-0596-7. PMID 12410385.
- ↑ "Correlation of amyloid fibril beta-structure with the unfolded state of alpha-synuclein". ChemBioChem 8 (14): 1671–1674. September 2007. doi:10.1002/cbic.200700366. PMID 17722123.
- ↑ "Conformational equilibria in monomeric alpha-synuclein at the single-molecule level". PLOS Biology 6 (1): e6. January 2008. doi:10.1371/journal.pbio.0060006. PMID 18198943.
- ↑ "The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia". Acta Neuropathologica 120 (2): 131–143. August 2010. doi:10.1007/s00401-010-0711-0. PMID 20563819.
- ↑ "The Inhibitory Effects of Cuminaldehyde on Amyloid Fibrillation and Cytotoxicity of Alpha-synuclein". Modares Journal of Medical Sciences: Pathobiology 15 (1): 45–60. Spring 2012.
- ↑ "Monoclonal antibodies against Epstein-Barr virus cross-react with alpha-synuclein in human brain". Neurology 55 (9): 1398–1401. November 2000. doi:10.1212/WNL.55.9.1398. PMID 11087792.
- ↑ "Mutation in the alpha-synuclein gene identified in families with Parkinson's disease". Science 276 (5321): 2045–2047. June 1997. doi:10.1126/science.276.5321.2045. PMID 9197268. https://zenodo.org/record/1231112.
- ↑ "Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease". Nature Genetics 18 (2): 106–108. February 1998. doi:10.1038/ng0298-106. PMID 9462735.
- ↑ "The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia". Annals of Neurology 55 (2): 164–173. February 2004. doi:10.1002/ana.10795. PMID 14755719.
- ↑ "Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease". Movement Disorders 28 (6): 811–813. June 2013. doi:10.1002/mds.25421. PMID 23457019.
- ↑ "G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome". Annals of Neurology 73 (4): 459–471. April 2013. doi:10.1002/ana.23894. PMID 23526723.
- ↑ "A new alpha-synuclein missense variant (Thr72Met) in two Turkish families with Parkinson's disease". Parkinsonism & Related Disorders 89: 63–72. August 2021. doi:10.1016/j.parkreldis.2021.06.023. PMID 34229155.
- ↑ "Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro". The Journal of Biological Chemistry 274 (12): 7619–7622. March 1999. doi:10.1074/jbc.274.12.7619. PMID 10075647.
- ↑ "Mutations associated with familial Parkinson's disease alter the initiation and amplification steps of α-synuclein aggregation". Proceedings of the National Academy of Sciences of the United States of America 113 (37): 10328–10333. September 2016. doi:10.1073/pnas.1604645113. PMID 27573854. Bibcode: 2016PNAS..11310328F.
- ↑ "alpha-Synuclein locus triplication causes Parkinson's disease". Science 302 (5646): 841. October 2003. doi:10.1126/science.1090278. PMID 14593171. https://zenodo.org/record/1230840.
- ↑ "Alpha-synuclein locus duplication as a cause of familial Parkinson's disease". Lancet 364 (9440): 1167–1169. 2004. doi:10.1016/S0140-6736(04)17103-1. PMID 15451224.
- ↑ "Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain". Brain 130 (Pt 3): 799–815. March 2007. doi:10.1093/brain/awl382. PMID 17303591.
- ↑ "Initiation and synergistic fibrillization of tau and alpha-synuclein". Science 300 (5619): 636–640. April 2003. doi:10.1126/science.1082324. PMID 12714745. Bibcode: 2003Sci...300..636G.
- ↑ "C-terminal alpha-synuclein immunoreactivity in structures other than Lewy bodies in neurodegenerative disorders". Acta Neuropathologica 99 (3): 296–304. March 2000. doi:10.1007/PL00007441. PMID 10663973.
- ↑ "Differential cross-seeding properties of tau and α-synuclein in mouse models of tauopathy and synucleinopathy". Brain Communications 2 (2): fcaa090. 2020-07-01. doi:10.1093/braincomms/fcaa090. PMID 33094280.
- ↑ "Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism". Proceedings of the National Academy of Sciences of the United States of America 112 (38): E5308–E5317. September 2015. doi:10.1073/pnas.1514475112. PMID 26324905. Bibcode: 2015PNAS..112E5308P.
- ↑ "New Type of Prion May Cause, Transmit Neurodegeneration". 31 August 2015. http://www.ucsf.edu/news/2015/08/131416/new-type-prion-may-cause-transmit-neurodegeneration.
- ↑ "Another Fatal Brain Disease May Come from the Spread of 'Prion' Proteins". Wired Science. 31 August 2015. http://www.livescience.com/52040-prions-multiple-system-atrophy.html.
- ↑ "alpha-Synuclein and neuronal cell death". Molecular Neurodegeneration 4 (1): 9. February 2009. doi:10.1186/1750-1326-4-9. PMID 19193223.
- ↑ "Novel self-replicating α-synuclein polymorphs that escape ThT monitoring can spontaneously emerge and acutely spread in neurons". Science Advances 6 (40): eabc4364. October 2020. doi:10.1126/sciadv.abc4364. PMID 33008896. Bibcode: 2020SciA....6.4364D.
- ↑ "alpha-Synuclein is phosphorylated in synucleinopathy lesions". Nature Cell Biology 4 (2): 160–164. February 2002. doi:10.1038/ncb748. PMID 11813001.
- ↑ "Attenuation of dopamine transporter activity by alpha-synuclein". Neuroscience Letters 340 (3): 189–192. April 2003. doi:10.1016/S0304-3940(03)00097-1. PMID 12672538.
- ↑ "Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis". FASEB Journal 15 (6): 916–926. April 2001. doi:10.1096/fj.00-0334com. PMID 11292651.
- ↑ "Co-association of parkin and alpha-synuclein". NeuroReport 12 (13): 2839–2843. September 2001. doi:10.1097/00001756-200109170-00017. PMID 11588587.
- ↑ "alpha-Synuclein aggregates interfere with Parkin solubility and distribution: role in the pathogenesis of Parkinson disease". The Journal of Biological Chemistry 283 (11): 6979–6987. March 2008. doi:10.1074/jbc.M710418200. PMID 18195004.
- ↑ "alpha-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells". The Journal of Biological Chemistry 277 (14): 12334–12342. April 2002. doi:10.1074/jbc.M110414200. PMID 11821392.
- ↑ "Analysis of synphilin-1 and synuclein interactions by yeast two-hybrid beta-galactosidase liquid assay". Neuroscience Letters 325 (2): 119–123. June 2002. doi:10.1016/S0304-3940(02)00253-7. PMID 12044636.
- ↑ "Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin". Cancer Research 51 (24): 6529–6538. December 1991. PMID 1742726.
- ↑ "Interaction of alpha-synuclein and synphilin-1: effect of Parkinson's disease-associated mutations". Journal of Neurochemistry 77 (3): 929–934. May 2001. doi:10.1046/j.1471-4159.2001.00301.x. PMID 11331421.
- ↑ "Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions". Nature Genetics 22 (1): 110–114. May 1999. doi:10.1038/8820. PMID 10319874.
- ↑ "More than just two peas in a pod: common amyloidogenic properties of tau and alpha-synuclein in neurodegenerative diseases". Trends in Neurosciences 27 (3): 129–134. March 2004. doi:10.1016/j.tins.2004.01.007. PMID 15036877.
- ↑ "alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356". The Journal of Biological Chemistry 274 (36): 25481–25489. September 1999. doi:10.1074/jbc.274.36.25481. PMID 10464279.
- ↑ "Interactions of amyloidogenic proteins". Neuromolecular Medicine 4 (1–2): 49–58. 2003. doi:10.1385/NMM:4:1-2:49. PMID 14528052.
- ↑ "Cross-seeding effects of amyloid β-protein and α-synuclein". Journal of Neurochemistry 122 (5): 883–890. September 2012. doi:10.1111/j.1471-4159.2012.07847.x. PMID 22734715. https://kanazawa-u.repo.nii.ac.jp/?action=repository_action_common_download&item_id=13927&item_no=1&attribute_id=26&file_no=1.
Further reading
- "In Folding Proteins, Clues to Many Diseases". New York Times. 2002-05-27. https://www.nytimes.com/2002/05/21/health/in-folding-proteins-clues-to-many-diseases.html.
- "Assessment of heterogeneity among participants in the Parkinson's Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study". Lancet Neurol 22 (5): 407–417. May 2023. doi:10.1016/S1474-4422(23)00109-6. PMID 37059509.
External links
- alpha-Synuclein at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human SNCA genome location and SNCA gene details page in the UCSC Genome Browser.
 |

