Biology:Organoid
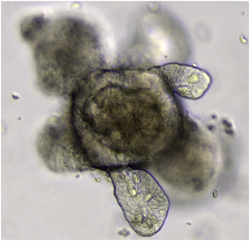
An organoid is a miniaturised and simplified version of an organ produced in vitro in three dimensions that mimics the key functional, structural and biological complexity of that organ.[1] They are derived from one or a few cells from a tissue, embryonic stem cells or induced pluripotent stem cells, which can self-organize in three-dimensional culture owing to their self-renewal and differentiation capacities. The technique for growing organoids has rapidly improved since the early 2010s, and The Scientist names it as one of the biggest scientific advancements of 2013.[2] Scientists and engineers use organoids to study development and disease in the laboratory, drug discovery and development in industry,[3] personalized diagnostics and medicine, gene and cell therapies, tissue engineering and regenerative medicine.
History
Attempts to create organs in vitro started with one of the first dissociation-reaggregation experiments[4] where Henry Van Peters Wilson demonstrated that mechanically dissociated sponge cells can reaggregate and self-organize to generate a whole organism.[5] In the subsequent decades, multiple labs were able to generate different types of organs[4] in vitro through the dissociation and reaggregation of organ tissues obtained from amphibians[6] and embryonic chicks.[7] The formation of first tissue-like colonies in vitro was observed for the first time by co-culturing keratinocytes and 3T3 fibroblasts.[8] The phenomena of mechanically dissociated cells aggregating and reorganizing to reform the tissue they were obtained from subsequently led to the development of the differential adhesion hypothesis by Malcolm Steinberg.[4] With the advent of the field of stem cell biology, the potential of stem cells to form organs in vitro was realized early on with the observation that when stem cells form teratomas or embryoid bodies, the differentiated cells can organize into different structures resembling those found in multiple tissue types.[4] The advent of the field of organoids, started with a shift from culturing and differentiating stem cells in two dimensional (2D) media, to three dimensional (3D) media to allow for the development of the complex 3-dimensional structures of organs.[4] Utilization of 3D media culture media methods for the structural organization was made possible with the development of extracellular matrices (ECM).[9] In the late 1980s, Bissell and colleagues showed that a laminin rich gel can be used as a basement membrane for differentiation and morphogenesis in cell cultures of mammary epithelial cells.[10][11] Since 1987, researchers have devised different methods for 3D culturing, and were able to utilize different types of stem cells to generate organoids resembling a multitude of organs.[4] In the 1990s, in addition to their role in physical support, the role of ECM components in gene expression by their interaction with integrin-based focal adhesion pathways was reported.[12] In 2006, Yaakov Nahmias and David Odde showed the self-assembly of vascular liver organoid maintained for over 50 days in vitro.[13] In 2008, Yoshiki Sasai and his team at RIKEN institute demonstrated that stem cells can be coaxed into balls of neural cells that self-organize into distinctive layers.[14] In 2009 the Laboratory of Hans Clevers at Hubrecht Institute and University Medical Center Utrecht, Netherlands, showed that single LGR5-expressing intestinal stem cells self-organize to crypt-villus structures in vitro without necessity of a mesenchymal niche, making them the first organoids.[15] In 2010, Mathieu Unbekandt & Jamie A. Davies demonstrated the production of renal organoids from murine fetus-derived renogenic stem cells.[16] In 2014, Qun Wang and co-workers engineered collagen-I and laminin based gels and synthetic foam biomaterials for the culture and delivery of intestinal organoids[17] and encapsulated DNA-functionalized gold nanoparticles into intestinal organoids to form an intestinal Trojan horse for drug delivery and gene therapy.[18] Subsequent reports showed significant physiological function of these organoids in vitro[19] and in vivo.[20][21]
Other significant early advancements included in 2013, Madeline Lancaster at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences established a protocol starting from pluripotent stem cells to generate cerebral organoids that mimic the developing human brain's cellular organization.[22] Meritxell Huch and Craig Dorrell at Hubrecht Institute and University Medical Center Utrecht demonstrated that single Lgr5+ cells from damaged mouse liver can be clonally expanded as liver organoids in Rspo1-based culture medium over several months.[23] In 2014, Artem Shkumatov et al. at the University of Illinois at Urbana-Champaign demonstrated that cardiovascular organoids can be formed from ES cells through modulation of the substrate stiffness, to which they adhere. Physiological stiffness promoted three-dimensionality of EBs and cardiomyogenic differentiation.[24] In 2015, Takebe et al. demonstrated a generalized method for organ bud formation from diverse tissues by combining pluripotent stem cell-derived tissue-specific progenitors or relevant tissue samples with endothelial cells and mesenchymal stem cells. They suggested that the less mature tissues, or organ buds, generated through the self-organized condensation principle might be the most efficient approach toward the reconstitution of mature organ functions after transplantation, rather than condensates generated from cells of a more advanced stage.[25]
Properties
Lancaster and Knoblich[4] define an organoid as a collection of organ-specific cell types that develops from stem cells or organ progenitors, self-organizes through cell sorting and spatially restricted lineage commitment in a manner similar to in vivo, and exhibits the following properties:
- it has multiple organ-specific cell types;
- it is capable of recapitulating some specific function of the organ (e.g. contraction, neural activity, endocrine secretion, filtration, excretion);
- its cells are grouped together and spatially organized, similar to an organ.
Process
Organoid formation generally requires culturing the stem cells or progenitor cells in a 3D medium.[4] Stem cells have the ability to self-renew and differentiate into various cell subtypes, and they enable understanding the processes of development and disease progression.[26] Therefore organoids derived from stem cells enable studying biology and physiology at the organ level.[27] The 3D medium can be made using an extracellular matrix hydrogel such as Matrigel or Cultrex BME, which is a laminin-rich extracellular matrix that is secreted by the Engelbreth-Holm-Swarm tumor line.[28] Organoid bodies can then be made through embedding stem cells in the 3D medium.[4] When pluripotent stem cells are used for the creation of the organoid, the cells are usually, but not all the time, allowed to form embryoid bodies.[4] Those embryoid bodies are then pharmacologically treated with patterning factors to drive the formation of the desired organoid identity.[4] Organoids have also been created using adult stem cells extracted from the target organ, and cultured in 3D media.[29]
Biochemical cues have been incorporated in 3D organoid cultures and with exposure of morphogenes, morphogen inhibitors, or growth factors, organoid models can be developed using embryonic stem cells (ESCs) or adult stem cells (ASCs). Vascularization techniques can be utilized to embody microenvironments that are close to their counterparts, physiologically. Vasculature systems that can facilitate oxygen or nutrients to the inner mass of organoids can be achieved through microfluidic systems, vascular endothelial growth factor delivery systems, and endothelial cell-coated modules.[9] With patient-derived induced pluripotent stem cells (iPSCs)[30] and CRISPR/Cas-based genome editing[31] technologies, genome-edited or mutated pluripotent stem cells (PSCs) with altered signaling cues can be generated to control intrinsic cues within organoids.
Types
A multitude of organ structures have been recapitulated using organoids.[4] This section aims to outline the state of the field as of now through providing an abridged list of the organoids that have been successfully created, along with a brief outline based on the most recent literature for each organoid, and examples of how it has been utilized in research.
Cerebral organoid
A cerebral organoid describes artificially grown, in vitro, miniature organs resembling the brain. Cerebral organoids are created by culturing human pluripotent stem cells in a three-dimensional structure using rotational bioreactor and develop over the course of months.[22] The procedure has potential applications in the study of brain development, physiology and function. Cerebral organoids may experience "simple sensations" in response to external stimulation and neuroscientists are among those expressing concern that such organs could develop sentience. They propose that further evolution of the technique needs to be subject to a rigorous oversight procedure.[32][33][34] In 2023, researchers have built a hybrid biocomputer that combines a laboratory-grown human brain organoids with conventional circuits, and can complete tasks such as voice recognition.[35] Cerebral Organoids are currently being used to research and develop Organoid Intelligence (OI) technologies.[36]
Gastrointestinal organoid
Gastrointestinal organoids refer to organoids that recapitulate structures of the gastrointestinal tract. The gastrointestinal tract arises from the endoderm, which during development forms a tube that can be divided in three distinct regions, which give rise to, along with other organs, the following sections of the gastrointestinal tract:[4]
- The foregut gives rise to the oral cavity and the stomach
- The midgut gives rise to the small intestines and the ascending colon
- The hindgut gives rise to the rectum and the rest of the colon
Organoids have been created for the following structures of the gastrointestinal tract:
Intestinal organoid
Intestinal organoids[15] have thus far been among the gut organoids generated directly from intestinal tissues or pluripotent stem cells.[4] One way human pluripotent stem cells can be driven to form intestinal organoids is through first the application of activin A to drive the cells into a mesoendodermal identity, followed by the pharmacological upregulation of Wnt3a and Fgf4 signaling pathways as they have been demonstrated to promote posterior gut fate.[4] Intestinal organoids have also been generated from intestinal stem cells, extracted from adult tissue and cultured in 3D media.[29] These adult stem cell-derived organoids are often referred to as enteroids or colonoids, depending on their segment of origin, and have been established from both the human and murine intestine.[15][37][38] Intestinal organoids consist of a single layer of polarized intestinal epithelial cells surrounding a central lumen. As such, recapitulate the crypt-villus structure of the intestine, by recapitulating its function, physiology and organization, and maintaining all the cell types found normally in the structure including intestinal stem cells.[4] Thus, intestinal organoids are a valuable model to study intestinal nutrient transport,[39][40] drug absorption and delivery,[41][42] nanomaterials and nanomedicine,[43][44] incretin hormone secretion,[45][46] and infection by various enteropathogens.[47][48] For example, Qun Wang's team rationally designed artificial virus nanoparticles as oral drug delivery vehicles (ODDVs) with gut organoid-derived mucosal models[49] and demonstrated a new concept of using newly established colon organoids as tools for high-throughput drug screening, toxicity testing, and oral drug development.[50] Intestinal organoids also recapitulate the crypt-Villus structure to such a high degree of fidelity that they have been successfully transplanted to mouse intestines, and are hence highly regarded as a valuable model for research.[4] One of the fields of research that intestinal organoids have been utilized is that of stem cell niche. Intestinal organoids were used to study the nature of the intestinal stem cell niche, and research done with them demonstrated the positive role IL-22 has in maintaining in intestinal stem cells,[51] along with demonstrating the roles of other cell types like neurons and fibroblasts in maintenance of intestinal stem cells.[29] In the field of infection biology, different intestinal organoid-based model systems have been explored. On one hand, organoids can be infected in bulk by simply mixing them with the enteropathogen of interest.[52] However, to model infection via a more natural route starting from the intestinal lumen, microinjection of the pathogen is required.[53][54] In addition, the polarity of intestinal organoids can be inverted,[55] and they can even be dissociated into single cells and cultured as 2D monolayers[56][57] in order to make both the apical and basolateral sides of the epithelium more easily accessible. Intestinal organoids have also demonstrated therapeutic potential.[58]

In order to more accurately recapitulate the intestine in vivo, co-cultures of intestinal organoids and immune cells have been developed.[57] Furthermore, organ-on-a-chip models combine intestinal organoids with other cell types such as endothelial or immune cells as well as peristaltic flow.[59][60]
Gastric organoid
Gastric organoids recapitulate at least partly the physiology of the stomach. Gastric organoids have been generated directly from pluripotent stem cells through the temporal manipulation of the FGF, WNT, BMP, retinoic acid and EGF signalling pathways in three-dimensional culture conditions.[61] Gastric organoids have also been generated using LGR5 expressing stomach adult stem cells.[62] Gastric organoids have been used as model for the study of cancer[63][64] along with human disease[61] and development.[61] For example, one study[64] investigated the underlying genetic alterations behind a patient's metastatic tumor population, and identified that unlike the patient's primary tumor, the metastasis had both alleles of the TGFBR2 gene mutated. To further assess the role of TGFBR2 in the metastasis, the investigators created organoids where TGFBR2 expression is knocked down, through which they were able to demonstrate that reduced TGFBR2 activity leads to invasion and metastasis of cancerous tumors both in vitro and in vivo.
Lingual organoid
Lingual organoids are organoids that recapitulate, at least partly, aspects of the tongue physiology. Epithelial lingual organoids have been generated using BMI1 expressing epithelial stem cells in three-dimensional culture conditions through the manipulation of EGF, WNT, and TGF-β.[65] This organoid culture, however, lacks taste receptors, as these cells do not arise from Bmi1 expressing epithelial stem cells.[65] Lingual taste bud organoids containing taste cells, however, have been created using the LGR5+ or CD44+ stem/progenitor cells of circumvallate (CV) papilla tissue.[66] These taste bud organoids have been successfully created both directly from isolated Lgr5- or LGR6-expressing taste stem/progenitor cells.[67] and indirectly, through the isolation, digestion, and subsequent culturing of CV tissue containing Lgr5+ or CD44+ stem/progenitor cells.[66]
Other
- Thymic organoids recapitulate at least partly the architecture and stem-cell niche functionality of the thymus,[70] which is a lymphoid organ where T cells mature. Thymic organoids have been generated through the seeding of thymic stromal cells in 3-dimensional culture.[70] Thymic organoids seem to successfully recapitulate the thymus' function, as co-culturing human hematopoietic or bone marrow stem cells with mouse thymic organoids resulted in the production of T-cells.[70]
- Testicular organoid[71]
- Prostate organoid[72]
- Hepatic organoid.[73] A recent study showed the usefulness of the technology for identifying novel medication for the treatment of hepatitis E as it allows to allows to recapitulate the entire viral life cycle.[74]
- Pancreatic organoid[75][76][77][78]
- Recent advances in cell repellent microtiter plates has allowed rapid, cost-effective screening of large small molecule drug like libraries against 3D models of pancreas cancer. These models are consistent in phenotype and expression profiles with those found in the lab of Dr. David Tuveson.
- Epithelial organoid[15][79]
- Lung organoid[80]
- Kidney organoid[16][81][82][83]
- Gastruloid (embryonic organoid)[84][85][86][87] – Generates all embryonic axes and fully implements the collinear Hox gene expression patterns along the anteroposterior axis.[87]
- Blastoid (blastocyst-like organoid)[88][89][90]
- Endometrial organoid[91]
- Cardiac organoid[92] – In 2018 hollow cardiac organoids were made to beat, and to respond to stimuli to beat faster or slower.[93]
- Retinal organoid[94][95]
- Breast cancer organoid[96]
- Colorectal cancer organoid[97]
- Glioblastoma organoid[98]
3D organoid models of brain cancer derived from either patient derived explants (PDX) or direct from cancer tissue is now easily achievable and affords high-throughput screening of these tumors against the current panel of approved drugs form around the world.
- Neuroendocrine tumor organoid[99]
- Myelinoid (Myelin organoid)
- Blood-brain barrier (BBB) organoid[100]
Self-assembled cell aggregates consisting of BMECs, astrocytes, and pericytes are emerging as a potential alternative to transwell and microfluidic models for certain applications. These organoides can generate many features of the BBB, such as the expression of tight junctions, molecular transporters, and drug efflux pumps, and can therefore be used to model drug transport across the BBB. Also, they can serve as a model for evaluating the interactions between the BBB and adjacent brain tissue and provide a platform for understanding the combined abilities of a new drug to overcome the BBB and its effect on brain tissue. In addition, such models are highly scalable and easier to manufacture and operate than microfluidic devices. However, they have limited ability to reconstruct the morphology and physiology of the BBB and are unable to simulate physiological flow and shear stress.
Basic research
Organoids enable to study how cells interact together in an organ, their interaction with their environment, how diseases affect them and the effect of drugs. In vitro culture makes this system easy to manipulate and facilitates their monitoring. While organs are difficult to culture because their size limits the penetration of nutrients, the small size of organoids limits this problem. On the other hand, they do not exhibit all organ features and interactions with other organs are not recapitulated in vitro. While research on stem cells and regulation of stemness was the first field of application of intestinal organoids,[15] they are now also used to study e.g. uptake of nutrients, drug transport and secretion of incretin hormones.[101] This is of great relevance in the context of malabsorption diseases as well as metabolic diseases such as obesity, insulin resistance, and diabetes.
Models of disease
Organoids provide an opportunity to create cellular models of human disease, which can be studied in the laboratory to better understand the causes of disease and identify possible treatments. The power of organoids in this regard was first shown for a genetic form of microcephaly, where patient cells were used to make cerebral organoids, which were smaller and showed abnormalities in early generation of neurons.[22] In another example, the genome editing system called CRISPR was applied to human pluripotent stem cells to introduce targeted mutations in genes relevant to two different kidney diseases, polycystic kidney disease and focal segmental glomerulosclerosis.[82] These CRISPR-modified pluripotent stem cells were subsequently grown into human kidney organoids, which exhibited disease-specific phenotypes. Kidney organoids from stem cells with polycystic kidney disease mutations formed large, translucent cyst structures from kidney tubules. When cultured in the absence of adherent cues (in suspension), these cysts reached sizes of 1 cm in diameter over several months.[102] Kidney organoids with mutations in a gene linked to focal segmental glomerulosclerosis developed junctional defects between podocytes, the filtering cells affected in that disease.[103] Importantly, these disease phenotypes were absent in control organoids of identical genetic background, but lacking the CRISPR mutations.[82][102][103] Comparison of these organoid phenotypes to diseased tissues from mice and humans suggested similarities to defects in early development.[102][103]
As first developed by Takahashi and Yamanaka in 2007, induced pluripotent stem cells (iPSC) can also be reprogrammed from patient skin fibroblasts.[104] These stem cells carry the exact genetic background of the patient including any genetic mutations which might contribute to the development of human disease. Differentiation of these cells into kidney organoids has been performed from patients with Lowe Syndrome due to ORCL1 mutations.[105] This report compared kidney organoids differentiated from patient iPSC to unrelated control iPSC and demonstrated an inability of patient kidney cells to mobilise transcription factor SIX2 from the golgi complex.[105] Because SIX2 is a well characterised marker of nephron progenitor cells in the cap mesenchyme, the authors concluded that renal disease frequently seen in Lowe Syndrome (global failure of proximal tubule reabsorption or renal Fanconi syndrome) could be related to alteration in nephron patterning arising from nephron progenitor cells lacking this important SIX2 gene expression.[105]
Other studies have used CRISPR gene editing to correct the patient's mutation in the patient iPSC cells to create an isogenic control, which can be performed simultaneously with iPSC reprogramming.[106][107][108] Comparison of a patient iPSC derived organoid against an isogenic control is the current gold standard in the field as it permits isolation of the mutation of interest as the only variable within the experimental model.[109] In one such report, kidney organoids derived from iPSC of a patient with Mainzer-Saldino Syndrome due to compound heterozygous mutations in IFT140 were compared to an isogenic control organoid in which an IFT140 variant giving rise to a non-viable mRNA transcript was corrected by CRISPR.[107] Patient kidney organoids demonstrated abnormal ciliary morphology consistent with existing animal models which was rescued to wild type morphology in the gene corrected organoids.[107] Comparative transcriptional profiling of epithelial cells purified from patient and control organoids highlighted pathways involved in cell polarity, cell-cell junctions and dynein motor assembly, some of which had been implicated for other genotypes within the phenotypic family of renal ciliopathies.[107] Another report utilising an isogenic control demonstrated abnormal nephrin localisation in the glomeruli of kidney organoids generated from a patient with congenital nephrotic syndrome.[108]
Things such as epithelial metabolism can also be modelled.[110]
Personalised medicine
Intestinal organoids grown from rectal biopsies using culture protocols established by the Clevers group have been used to model cystic fibrosis,[111] and led to the first application of organoids for personalised treatment.[112] Cystic fibrosis is an inherited disease that is caused by gene mutations of the cystic fibrosis transmembrane conductance regulator gene that encodes an epithelial ion channel necessary for healthy epithelial surface fluids. Studies by the laboratory of Jeffrey Beekman (Wilhelmina Children's Hospital, University Medical Center Utrecht, The Netherlands) described in 2013 that stimulation of colorectal organoids with cAMP-raising agonists such as forskolin or cholera toxin induced rapid swelling of organoids in a fully CFTR dependent manner.[111] Whereas organoids from non-cystic fibrosis subjects swell in response to forskolin as a consequence of fluid transport into the organoids' lumens, this is severely reduced or absent in organoids derived from people with cystic fibrosis. Swelling could be restored by therapeutics that repair the CFTR protein (CFTR modulators), indicating that individual responses to CFTR modulating therapy could be quantitated in a preclinical laboratory setting. Schwank et al. also demonstrated that the intestinal cystic fibrosis organoid phenotype could be repaired by CRISPR-Cas9 gene editing in 2013.[113]
Follow-up studies by Dekkers et al. in 2016 revealed that quantitative differences in forskolin-induced swelling between intestinal organoids derived from people with cystic fibrosis associate with known diagnostic and prognostic markers such as CFTR gene mutations or in vivo biomarkers of CFTR function.[112] In addition, the authors demonstrated that CFTR modulator responses in intestinal organoids with specific CFTR mutations correlated with published clinical trial data of these treatments. This led to preclinical studies where organoids from patients with extremely rare CFTR mutations for who no treatment was registered were found to respond strongly to a clinically available CFTR modulator. The suggested clinical benefit of treatment for these subjects based on the preclinical organoid test was subsequently confirmed upon clinical introduction of treatment by members of the clinical CF center under supervision of Kors van der Ent (Department of Paediatric Pulmonology, Wilhelmina Children's Hospital, University Medical Center Utrecht, The Netherlands). These studies show for the first time that organoids can be used for the individual tailoring of therapy or personalised medicine.
Organoid transplants
The first successful transplantation of an organoid into a human, a patient with ulcerative colitis whose cells were used for the organoid, was carried out in 2022.[114][115]
As a model for developmental biology
Organoids offer researchers an exceptional model to study developmental biology.[116] Since the identification of pluripotent stem cells, there have been great advancements in directing pluripotent stem cells fate in vitro using 2D cultures.[116] These advancements in PSC fate direction, coupled with the advancements in 3D culturing techniques allowed for the creation of organoids that recapitulate the properties of various specific subregions of a multitude of organs.[116] The use of these organoids has thus greatly contributed to expanding our understanding of the processes of organogenesis, and the field of developmental biology.[116] In central nervous system development, for example, organoids have contributed to our understanding of the physical forces that underlie retinal cup formation.[116][117] More recent work has extended cortical organoid growth periods extensively and at nearly a year under specific differentiation conditions, the organoids persist and have some features of human fetal development stages.[118]
See also
References
- ↑ Zhao Z, Chen X, Dowbaj AM, Sljukic A, Bratlie K, Lin L, Fong ELS, Balachander GM, Chen Z, Soragni A, Huch M, Zeng YA, Wang Q, Yu H. Organoids. Nature Reviews Methods Primers 2, 94 (2022). https://doi.org/10.1038/s43586-022-00174-y
- ↑ Grens, Kerry (December 24, 2013). "2013's Big Advances in Science". http://www.the-scientist.com/?articles.view/articleNo/38747/title/2013-s-Big-Advances-in-Science/.
- ↑ Hans Clevers, Asher Mullard. Mini-organs attract big pharma. Nature Reviews Drug Discovery (16 February 2023). https://doi.org/10.1038/d41573-023-00030-y
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 "Organogenesis in a dish: modeling development and disease using organoid technologies". Science 345 (6194): 1247125. July 2014. doi:10.1126/science.1247125. PMID 25035496.
- ↑ "A new method by which sponges may be artificially reared". Science 25 (649): 912–5. June 1907. doi:10.1126/science.25.649.912. PMID 17842577. Bibcode: 1907Sci....25..912W. https://zenodo.org/record/1447988.
- ↑ "Experimental studies on the development of the pronephros.". Rev. Can. Biol. 3: 220–250. 1944.
- ↑ "Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation". Proceedings of the National Academy of Sciences of the United States of America 46 (9): 1177–85. September 1960. doi:10.1073/pnas.46.9.1177. PMID 16590731. Bibcode: 1960PNAS...46.1177W.
- ↑ Rheinwald, James G.; Green, Howard (November 1975). "Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma". Cell 6 (3): 317–330. doi:10.1016/0092-8674(75)90183-x. ISSN 0092-8674. PMID 1052770. http://dx.doi.org/10.1016/0092-8674(75)90183-x.
- ↑ 9.0 9.1 Yi, Sang Ah; Zhang, Yixiao; Rathnam, Christopher; Pongkulapa, Thanapat; Lee, Ki-Bum (November 2021). "Bioengineering Approaches for the Advanced Organoid Research" (in en). Advanced Materials 33 (45): e2007949. doi:10.1002/adma.202007949. ISSN 0935-9648. PMID 34561899. Bibcode: 2021AdM....3307949Y.
- ↑ Li, M L; Aggeler, J; Farson, D A; Hatier, C; Hassell, J; Bissell, M J (January 1987). "Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells.". Proceedings of the National Academy of Sciences 84 (1): 136–140. doi:10.1073/pnas.84.1.136. ISSN 0027-8424. PMID 3467345. Bibcode: 1987PNAS...84..136L.
- ↑ Barcellos-Hoff, M. H.; Aggeler, J.; Ram, T. G.; Bissell, M. J. (1989-02-01). "Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane". Development 105 (2): 223–235. doi:10.1242/dev.105.2.223. ISSN 0950-1991. PMID 2806122. PMC 2948482. http://dx.doi.org/10.1242/dev.105.2.223.
- ↑ Streuli, C H; Schmidhauser, C; Bailey, N; Yurchenco, P; Skubitz, A P; Roskelley, C; Bissell, M J (1995-05-01). "Laminin mediates tissue-specific gene expression in mammary epithelia.". The Journal of Cell Biology 129 (3): 591–603. doi:10.1083/jcb.129.3.591. ISSN 0021-9525. PMID 7730398. PMC 2120432. http://dx.doi.org/10.1083/jcb.129.3.591.
- ↑ "Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro". Tissue Engineering 12 (6): 1627–38. June 2006. doi:10.1089/ten.2006.12.1627. PMID 16846358.
- ↑ Yong, Ed (August 28, 2013). "Lab-Grown Model Brains". http://www.the-scientist.com/?articles.view/articleNo/37262/title/Lab-Grown-Model-Brains/.
- ↑ 15.0 15.1 15.2 15.3 15.4 "Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche". Nature 459 (7244): 262–5. May 2009. doi:10.1038/nature07935. PMID 19329995. Bibcode: 2009Natur.459..262S.
- ↑ 16.0 16.1 "Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues". Kidney International 77 (5): 407–16. March 2010. doi:10.1038/ki.2009.482. PMID 20016472.
- ↑ Peng H, Poovaiah N, Forrester M, Cochran E, Wang Q. Ex Vivo Culture of Primary Intestinal Stem Cells in Collagen Gels and Foams. ACS Biomaterials Science & Engineering. 2014 Dec 2;1(1):37–42. https://doi.org/10.1021/ab500041d. PMID: 33435081.
- ↑ Peng H, Wang C, Xu X, Yu C, Wang Q. An intestinal Trojan horse for gene delivery. Nanoscale. 2015 Jan 6;7(10):4354–4360. https://doi.org/10.1039/C4NR06377E. PMID: 25619169.
- ↑ "Transport of organic anions and cations in murine embryonic kidney development and in serially-reaggregated engineered kidneys". Scientific Reports 5: 9092. March 2015. doi:10.1038/srep09092. PMID 25766625. Bibcode: 2015NatSR...5E9092L.
- ↑ "In vivo maturation of functional renal organoids formed from embryonic cell suspensions". Journal of the American Society of Nephrology 23 (11): 1857–68. November 2012. doi:10.1681/ASN.2012050505. PMID 23085631.
- ↑ Yui, S., Nakamura, T., Sato, T. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nature Medicine 18, 618–623 (2012). https://doi.org/10.1038/nm.2695
- ↑ 22.0 22.1 22.2 "Cerebral organoids model human brain development and microcephaly". Nature 501 (7467): 373–9. September 2013. doi:10.1038/nature12517. PMID 23995685. Bibcode: 2013Natur.501..373L.
- ↑ Huch, M., Dorrell, C., Boj, S. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013). https://doi.org/10.1038/nature11826
- ↑ "Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies". PLOS ONE 9 (4): e94764. 2014. doi:10.1371/journal.pone.0094764. PMID 24732893. Bibcode: 2014PLoSO...994764S.
- ↑ "Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation". Cell Stem Cell 16 (5): 556–65. May 2015. doi:10.1016/j.stem.2015.03.004. PMID 25891906.
- ↑ Murry, Charles E.; Keller, Gordon (February 2008). "Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development". Cell 132 (4): 661–680. doi:10.1016/j.cell.2008.02.008. ISSN 0092-8674. PMID 18295582. http://dx.doi.org/10.1016/j.cell.2008.02.008.
- ↑ Choudhury, Deepak; Ashok, Aswathi; Naing, May Win (March 2020). "Commercialization of Organoids". Trends in Molecular Medicine 26 (3): 245–249. doi:10.1016/j.molmed.2019.12.002. ISSN 1471-4914. PMID 31982341. http://dx.doi.org/10.1016/j.molmed.2019.12.002.
- ↑ "Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells". Proceedings of the National Academy of Sciences of the United States of America 84 (1): 136–40. January 1987. doi:10.1073/pnas.84.1.136. PMID 3467345. Bibcode: 1987PNAS...84..136L.
- ↑ 29.0 29.1 29.2 "Three-Dimensional Gastrointestinal Organoid Culture in Combination with Nerves or Fibroblasts: A Method to Characterize the Gastrointestinal Stem Cell Niche". Stem Cells International 2016: 3710836. 2016. doi:10.1155/2016/3710836. PMID 26697073.
- ↑ Takahashi, Kazutoshi; Yamanaka, Shinya (August 2006). "Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors". Cell 126 (4): 663–676. doi:10.1016/j.cell.2006.07.024. ISSN 0092-8674. PMID 16904174. http://dx.doi.org/10.1016/j.cell.2006.07.024.
- ↑ Ran, F Ann; Hsu, Patrick D; Wright, Jason; Agarwala, Vineeta; Scott, David A; Zhang, Feng (2013-10-24). "Genome engineering using the CRISPR-Cas9 system". Nature Protocols 8 (11): 2281–2308. doi:10.1038/nprot.2013.143. ISSN 1754-2189. PMID 24157548. PMC 3969860. http://dx.doi.org/10.1038/nprot.2013.143.
- ↑ "Cerebral organoids: ethical issues and consciousness assessment". Journal of Medical Ethics 44 (9): 606–610. September 2018. doi:10.1136/medethics-2017-104555. PMID 29491041.
- ↑ Prosser Scully, Ruby (6 July 2019). "Miniature brains grown in the lab have human-like neural activity". New Scientist (3237). https://www.newscientist.com/article/2207911-miniature-brains-grown-in-the-lab-have-human-like-neural-activity/.
- ↑ Sample, Ian (21 October 2019). "Scientists 'may have crossed ethical line' in growing human brains". The Guardian: p. 15. https://www.theguardian.com/science/2019/oct/21/scientists-may-have-crossed-ethical-line-in-growing-human-brains.
- ↑ Cai, H., Ao, Z., Tian, C. et al. Brain organoid reservoir computing for artificial intelligence. Nat Electron (2023). https://doi.org/10.1038/s41928-023-01069-w.
- ↑ Smirnova L, Caffo BS, Gracias DH, Huang Q, Morales Pantoja IE, Tang B, Zack DJ, Berlinicke CA, Boyd JL, Harris TD, Johnson EC, Kagan BJ, Kahn J, Muotri AR, Paulhamus BL, Schwamborn JC, Plotkin J, Szalay AS, Vogelstein JT, Worley PF and Hartung T. Organoid intelligence (OI): the new frontier in biocomputing and intelligence-in-a-dish. Front Sci (2023) 1:1017235. https://doi.org/10.3389/fsci.2023.1017235.
- ↑ Sato, Toshiro; Stange, Daniel E.; Ferrante, Marc; Vries, Robert G.J.; van Es, Johan H.; van den Brink, Stieneke; van Houdt, Winan J.; Pronk, Apollo et al. (November 2011). "Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett's Epithelium". Gastroenterology 141 (5): 1762–1772. doi:10.1053/j.gastro.2011.07.050. ISSN 0016-5085. PMID 21889923.
- ↑ Jung, Peter; Sato, Toshiro; Merlos-Suárez, Anna; Barriga, Francisco M; Iglesias, Mar; Rossell, David; Auer, Herbert; Gallardo, Mercedes et al. (October 2011). "Isolation and in vitro expansion of human colonic stem cells" (in en). Nature Medicine 17 (10): 1225–1227. doi:10.1038/nm.2470. ISSN 1078-8956. PMID 21892181. http://www.nature.com/articles/nm.2470.
- ↑ Cai T, Qi Y, Jergens A, Wannemuehler M, Barrett TA, Wang Q. Effects of six common dietary nutrients on murine intestinal organoid growth. PLoS One. 2018 Feb 1;13(2):e0191517. https://doi.org/10.1371/journal.pone.0191517. PMID: 29389993; PMCID: PMC5794098.
- ↑ Qi Y, Lohman J, Bratlie KM, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Yoon KJ, Barrett TA, Wang Q. Vitamin C and B3 as new biomaterials to alter intestinal stem cells. Journal of Biomedical Materials Research Part A. 2019 Sep;107(9):1886–1897. https://doi.org/10.1002/jbm.a.36715. Epub 2019 May 23. PMID: 31071241; PMCID: PMC6626554.
- ↑ Davoudi Z, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Barrett TA, Narasimhan B, Wang Q. Intestinal organoids containing poly(lactic-co-glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. Journal of Biomedical Materials Research Part A. 2018 Apr;106(4):876–886. https://doi.org/10.1002/jbm.a.36305. Epub 2017 Dec 21. PMID: 29226615; PMCID: PMC5826879.
- ↑ Davoudi Z, Peroutka-Bigus N, Bellaire B, Jergens A, Wannemuehler M, Wang Q. Gut Organoid as a New Platform to Study Alginate and Chitosan Mediated PLGA Nanoparticles for Drug Delivery. Marine Drugs. 2021 May 20;19(5):282. https://doi.org/10.3390/md19050282. PMID: 34065505; PMCID: PMC8161322.
- ↑ Qi Y, Shi E, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Jergens A, Barrett T, Wu Y, Wang Q. Ex Vivo Study of Telluride Nanowires in Minigut. Journal of Biomedical Nanotechnology. 2018 May 1;14(5):978–986. https://doi.org/10.1166/jbn.2018.2578. PMID: 29883567
- ↑ Reding B, Carter P, Qi Y, Li Z, Wu Y, Wannemuehler M, Bratlie KM, Wang Q. Manipulate intestinal organoids with niobium carbide nanosheets. Journal of Biomedical Materials Research Part A. 2021 Apr;109(4):479–487. https://doi.org/10.1002/jbm.a.37032. Epub 2020 Jun 17. PMID: 32506610.
- ↑ "Organoids to Study Intestinal Nutrient Transport, Drug Uptake and Metabolism – Update to the Human Model and Expansion of Applications". Frontiers in Bioengineering and Biotechnology 8: 577656. 2020. doi:10.3389/fbioe.2020.577656. PMID 33015026.
- ↑ "Intestinal organoids for assessing nutrient transport, sensing and incretin secretion". Scientific Reports 5 (1): 16831. November 2015. doi:10.1038/srep16831. PMID 26582215. Bibcode: 2015NatSR...516831Z.
- ↑ Rahmani, Sara; Breyner, Natalia M.; Su, Hsuan-Ming; Verdu, Elena F.; Didar, Tohid F. (2019-02-01). "Intestinal organoids: A new paradigm for engineering intestinal epithelium in vitro" (in en). Biomaterials 194: 195–214. doi:10.1016/j.biomaterials.2018.12.006. ISSN 0142-9612. PMID 30612006. https://www.sciencedirect.com/science/article/abs/pii/S0142961218308287.
- ↑ Sun L, Rollins D, Qi Y, Fredericks J, Mansell TJ, Jergens A, Phillips GJ, Wannemuehler M, Wang Q. TNFα regulates intestinal organoids from mice with both defined and conventional microbiota. International Journal of Biological Macromolecules. 2020 Dec 1;164:548–556. https://doi.org/10.1016/j.ijbiomac.2020.07.176. Epub 2020 Jul 18. PMID: 32693143; PMCID: PMC7657954.
- ↑ Tong T, Qi Y, Rollins D, Bussiere LD, Dhar D, Miller CL, Yu C, Wang Q. Rational design of oral drugs targeting mucosa delivery with gut organoid platforms. Bioactive Materials. 2023; 30: 116–128. https://doi.org/10.1016/j.bioactmat.2023.07.014. PMID: 37560199.
- ↑ Davoudi Z, Atherly T, Borcherding DC, Jergens AE, Wannemuehler M, Barrett TA, Wang Q. Study Transportation of Drugs within Newly Established Murine Colon Organoid Systems. Advanced Biology. 2023; e2300103. https://doi.org/10.1002/adbi.202300103. PMID: 37607116.
- ↑ "IL-22 Administration Protects Intestinal Stem Cells from Gvhd". Biology of Blood and Marrow Transplantation 20 (2): S53–S54. 2014. doi:10.1016/j.bbmt.2013.12.056.
- ↑ Zhang, Yong-Guo; Wu, Shaoping; Xia, Yinglin; Sun, Jun (September 2014). "Salmonella -infected crypt-derived intestinal organoid culture system for host-bacterial interactions" (in en). Physiological Reports 2 (9): e12147. doi:10.14814/phy2.12147. PMID 25214524.
- ↑ Geiser, Petra; Di Martino, Maria Letizia; Samperio Ventayol, Pilar; Eriksson, Jens; Sima, Eduardo; Al-Saffar, Anas Kh.; Ahl, David; Phillipson, Mia et al. (2021-02-23). Sperandio, Vanessa. ed. "Salmonella enterica Serovar Typhimurium Exploits Cycling through Epithelial Cells To Colonize Human and Murine Enteroids" (in en). mBio 12 (1). doi:10.1128/mBio.02684-20. ISSN 2161-2129. PMID 33436434.
- ↑ Dutta, Devanjali; Heo, Inha; O'Connor, Roberta (2019-09-14). "Studying Cryptosporidium Infection in 3D Tissue-derived Human Organoid Culture Systems by Microinjection" (in en). Journal of Visualized Experiments (151): 59610. doi:10.3791/59610. ISSN 1940-087X. PMID 31566619. https://www.jove.com/video/59610/studying-cryptosporidium-infection-3d-tissue-derived-human-organoid.
- ↑ Co, Julia Y.; Margalef-Català, Mar; Li, Xingnan; Mah, Amanda T.; Kuo, Calvin J.; Monack, Denise M.; Amieva, Manuel R. (February 2019). "Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions" (in en). Cell Reports 26 (9): 2509–2520.e4. doi:10.1016/j.celrep.2019.01.108. PMID 30811997.
- ↑ Tong T , Qi Y , Bussiere LD , Wannemuehler M , Miller CL , Wang Q , Yu C . Transport of artificial virus-like nanocarriers through intestinal monolayers via microfold cells. Nanoscale. 2020 Aug 14;12(30):16339-16347. https://doi.org/10.1039/D0NR03680C. Epub 2020 Jul 29. PMID: 32725029.
- ↑ 57.0 57.1 Noel, Gaelle; Baetz, Nicholas W.; Staab, Janet F.; Donowitz, Mark; Kovbasnjuk, Olga; Pasetti, Marcela F.; Zachos, Nicholas C. (2017-05-31). "A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions" (in en). Scientific Reports 7 (1): 45270. doi:10.1038/srep45270. ISSN 2045-2322. PMID 28345602. Bibcode: 2017NatSR...745270N.
- ↑ "FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures". Nature Communications 5: 4242. June 2014. doi:10.1038/ncomms5242. PMID 24979718. Bibcode: 2014NatCo...5.4242B.
- ↑ Sontheimer-Phelps, Alexandra; Chou, David B.; Tovaglieri, Alessio; Ferrante, Thomas C.; Duckworth, Taylor; Fadel, Cicely; Frismantas, Viktoras; Sutherland, Arlene D. et al. (2020). "Human Colon-on-a-Chip Enables Continuous In Vitro Analysis of Colon Mucus Layer Accumulation and Physiology" (in en). Cellular and Molecular Gastroenterology and Hepatology 9 (3): 507–526. doi:10.1016/j.jcmgh.2019.11.008. PMID 31778828.
- ↑ Grassart, Alexandre; Malardé, Valérie; Gobaa, Samy; Sartori-Rupp, Anna; Kerns, Jordan; Karalis, Katia; Marteyn, Benoit; Sansonetti, Philippe et al. (September 2019). "Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection" (in en). Cell Host & Microbe 26 (3): 435–444.e4. doi:10.1016/j.chom.2019.08.007. PMID 31492657.
- ↑ 61.0 61.1 61.2 "Modelling human development and disease in pluripotent stem-cell-derived gastric organoids". Nature 516 (7531): 400–4. December 2014. doi:10.1038/nature13863. PMID 25363776. Bibcode: 2014Natur.516..400M.
- ↑ "Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro". Cell Stem Cell 6 (1): 25–36. January 2010. doi:10.1016/j.stem.2009.11.013. PMID 20085740.
- ↑ "Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture". Nature Medicine 20 (7): 769–77. July 2014. doi:10.1038/nm.3585. PMID 24859528.
- ↑ 64.0 64.1 "Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer". Genome Biology 15 (8): 428. August 2014. doi:10.1186/s13059-014-0428-9. PMID 25315765.
- ↑ 65.0 65.1 "Establishment of a novel lingual organoid culture system: generation of organoids having mature keratinized epithelium from adult epithelial stem cells". Scientific Reports 3: 3224. November 2013. doi:10.1038/srep03224. PMID 24232854. Bibcode: 2013NatSR...3E3224H.
- ↑ 66.0 66.1 "Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid". Scientific Reports 5: 17185. November 2015. doi:10.1038/srep17185. PMID 26597788. Bibcode: 2015NatSR...517185A.
- ↑ "Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo". Proceedings of the National Academy of Sciences of the United States of America 111 (46): 16401–6. November 2014. doi:10.1073/pnas.1409064111. PMID 25368147. Bibcode: 2014PNAS..11116401R.
- ↑ "T-cell receptors and autoimmune thyroid disease—signposts for T-cell-antigen driven diseases". International Reviews of Immunology 18 (1–2): 111–40. 1999. doi:10.3109/08830189909043021. PMID 10614741.
- ↑ "An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts". Nature Cell Biology 16 (9): 902–8. September 2014. doi:10.1038/ncb3023. PMID 25150981.
- ↑ 70.0 70.1 70.2 Generation of a tissue-engineered thymic organoid. Methods in Molecular Biology. 380. 2007. pp. 163–70. doi:10.1385/1-59745-395-1:163. ISBN 978-1-59745-395-0.
- ↑ Sakib, Sadman (1 June 2019). "Formation of organotypic testicular organoids in microwell culture". Biology of Reproduction 100 (6): 1648–1660. doi:10.1093/biolre/ioz053. PMID 30927418.
- ↑ Drost, Jarno; Karthaus, Wouter R.; Gao, Dong; Driehuis, Else; Sawyers, Charles L.; Chen, Yu; Clevers, Hans (21 January 2016). "Organoid culture systems for prostate epithelial and cancer tissue" (in en). Nature Protocols 11 (2): 347–358. doi:10.1038/nprot.2016.006. ISSN 1750-2799. PMID 26797458.
- ↑ "Long-term culture of genome-stable bipotent stem cells from adult human liver". Cell 160 (1–2): 299–312. January 2015. doi:10.1016/j.cell.2014.11.050. PMID 25533785.
- ↑ Li P, Li Y, Wang Y, Liu J, Lavrijsen M, Li Y, Zhang R, Verstegen MMA, Wang Y, Li TC, Ma Z, Kainov DE, Bruno MJ, de Man RA, van der Laan LJW, Peppelenbosch MP, Pan Q (2022). "Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids". Science Advances 8 (3): 103–111. doi:10.1126/sciadv.abj5908. PMID 5044825. Bibcode: 2022SciA....8.5908L. https://pure.eur.nl/en/publications/2a130980-ce88-4339-934b-df2aef36716c.
- ↑ "Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis". The EMBO Journal 32 (20): 2708–21. October 2013. doi:10.1038/emboj.2013.204. PMID 24045232.
- ↑ Hou S, Tiriac H, Sridharan BP, Scampavia L, Madoux F, Seldin J (2018). "Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening.". SLAS Discov 23 (6): 574–584. doi:10.1177/2472555218766842. PMID 29673279.
- ↑ Wolff RA, Wang-Gillam A, Alvarez H, Tiriac H, Engle D, Hou S (2018). "Dynamic changes during the treatment of pancreatic cancer.". Oncotarget 9 (19): 14764–14790. doi:10.18632/oncotarget.24483. PMID 29599906.
- ↑ Below, Christopher R.; Kelly, Joanna; Brown, Alexander; Humphries, Jonathan D.; Hutton, Colin; Xu, Jingshu; Lee, Brian Y.; Cintas, Celia et al. (2021-09-13). "A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids" (in en). Nature Materials 21 (1): 110–119. doi:10.1038/s41563-021-01085-1. ISSN 1476-4660. PMID 34518665.
- ↑ "Identification of stem cells in small intestine and colon by marker gene Lgr5". Nature 449 (7165): 1003–7. October 2007. doi:10.1038/nature06196. PMID 17934449. Bibcode: 2007Natur.449.1003B.
- ↑ "Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis". Cell 156 (3): 440–55. January 2014. doi:10.1016/j.cell.2013.12.039. PMID 24485453.
- ↑ "Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis". Nature 526 (7574): 564–8. October 2015. doi:10.1038/nature15695. PMID 26444236. Bibcode: 2015Natur.526..564T.
- ↑ 82.0 82.1 82.2 "Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids". Nature Communications 6: 8715. October 2015. doi:10.1038/ncomms9715. PMID 26493500. Bibcode: 2015NatCo...6.8715F.
- ↑ "Nephron organoids derived from human pluripotent stem cells model kidney development and injury". Nature Biotechnology 33 (11): 1193–200. November 2015. doi:10.1038/nbt.3392. PMID 26458176.
- ↑ "Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells". Development 141 (22): 4231–42. November 2014. doi:10.1242/dev.113001. PMID 25371360.
- ↑ "Organoids and the genetically encoded self-assembly of embryonic stem cells". BioEssays 38 (2): 181–91. February 2016. doi:10.1002/bies.201500111. PMID 26666846.
- ↑ "Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids". Development 144 (21): 3894–3906. November 2017. doi:10.1242/dev.150391. PMID 28951435.
- ↑ 87.0 87.1 "Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids" (in En). Nature 562 (7726): 272–276. October 2018. doi:10.1038/s41586-018-0578-0. PMID 30283134. Bibcode: 2018Natur.562..272B. https://www.repository.cam.ac.uk/handle/1810/285960.
- ↑ "Blastoid: The backstory of the formation of blastocyst-like structure solely from stem cells". 2018-06-27. https://thenode.biologists.com/blastoid-the-backstory-of-the-formation-of-blastocyst-like-structure-solely-from-stem-cells/highlights/.
- ↑ "Nicolas Rivron Lab | Blastoid | Netherlands". https://www.nicolasrivron.org/theblastoid.
- ↑ "Blastocyst-like structures generated solely from stem cells". Nature 557 (7703): 106–111. May 2018. doi:10.1038/s41586-018-0051-0. PMID 29720634. Bibcode: 2018Natur.557..106R. https://cris.maastrichtuniversity.nl/ws/files/60703351/Blitterswijk_2018_Blastocyst_like_structures_generated_solely.pdf.
- ↑ "Organoids to model the endometrium: implantation and beyond". Reprod Fertil 2 (3): R85–R101. July 2021. doi:10.1530/RAF-21-0023. PMID 35118399.
- ↑ "Engineered cardiac organoid chambers: toward a functional biological model ventricle". Tissue Engineering. Part A 14 (2): 215–25. February 2008. doi:10.1089/tea.2007.0351. PMID 18333774.
- ↑ Molteni, Megan (2018-06-27). "These Beating Mini-Hearts Could Save Big Bucks—And Maybe Lives". WIRED. https://www.wired.com/story/these-beating-mini-hearts-could-save-big-bucksand-maybe-lives/. Retrieved 2018-06-30.
- ↑ "cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness". Scientific Reports 6: 30742. July 2016. doi:10.1038/srep30742. PMID 27471043. Bibcode: 2016NatSR...630742W.
- ↑ Zilova, Lucie; Weinhardt, Venera; Tavhelidse, Tinatini; Schlagheck, Christina; Thumberger, Thomas; Wittbrodt, Joachim (2021-07-12). Martínez Arias, Alfonso; Stainier, Didier YR; Martínez Arias, Alfonso. eds. "Fish primary embryonic pluripotent cells assemble into retinal tissue mirroring in vivo early eye development". eLife 10: e66998. doi:10.7554/eLife.66998. ISSN 2050-084X. PMID 34252023.
- ↑ Sachs, Norman; de Ligt, Joep; Kopper, Oded; Gogola, Ewa; Bounova, Gergana; Weeber, Fleur; Balgobind, Anjali Vanita; Wind, Karin et al. (2018). "A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity". Cell 172 (1–2): 373–386.e10. doi:10.1016/j.cell.2017.11.010. ISSN 0092-8674. PMID 29224780.
- ↑ van de Wetering, Marc; Francies, Hayley; Francis, Joshua; Bounova, Gergana; Iorio, Francesco; Pronk, Apollo; van Houdt, Winan; van Gorp, Joost et al. (2015). "Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients". Cell 161 (4): 933–945. doi:10.1016/j.cell.2015.03.053. ISSN 0092-8674. PMID 25957691. PMC 6428276. https://doi.org/10.1016/j.cell.2015.03.053.
- ↑ Quereda V, Hou S, Madoux F, Scampavia L, Spicer TP, Duckett D (2018). "A Cytotoxic Three-Dimensional-Spheroid, High-Throughput Assay Using Patient-Derived Glioma Stem Cells.". SLAS Discov 23 (8): 842–849. doi:10.1177/2472555218775055. PMID 29750582.
- ↑ Dayton, Talya L.; Alcala, Nicolas; Moonen, Laura; den Hartigh, Lisanne; Geurts, Veerle; Mangiante, Lise; Lap, Lisa; Dost, Antonella F.M. et al. (2023). "Druggable growth dependencies and tumor evolution analysis in patient-derived organoids of neuroendocrine neoplasms from multiple body sites". Cancer Cell 41 (12): 2083–2099. doi:10.1016/j.ccell.2023.11.007. ISSN 1535-6108. PMID 38086335.
- ↑ Zidarič, Tanja; Gradišnik, Lidija; Velnar, Tomaž (2022-04-01). "Astrocytes and human artificial blood-brain barrier models" (in en). Bosnian Journal of Basic Medical Sciences 22 (5): 651–672. doi:10.17305/bjbms.2021.6943. ISSN 1840-4812. PMID 35366791. PMC 9519155. https://www.bjbms.org/ojs/index.php/bjbms/article/view/6943.
- ↑ "Intestinal organoids for assessing nutrient transport, sensing and incretin secretion". Scientific Reports 5: 16831. November 2015. doi:10.1038/srep16831. PMID 26582215. Bibcode: 2015NatSR...516831Z.
- ↑ 102.0 102.1 102.2 "Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease". Nature Materials 16 (11): 1112–1119. November 2017. doi:10.1038/nmat4994. PMID 28967916. Bibcode: 2017NatMa..16.1112C.
- ↑ 103.0 103.1 103.2 "Gene-Edited Human Kidney Organoids Reveal Mechanisms of Disease in Podocyte Development". Stem Cells 35 (12): 2366–2378. December 2017. doi:10.1002/stem.2707. PMID 28905451.
- ↑ "Induction of pluripotent stem cells from adult human fibroblasts by defined factors". Cell 131 (5): 861–72. November 2007. doi:10.1016/j.cell.2007.11.019. PMID 18035408. https://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/49782/1/Yamanaka_Cell_131_5.pdf.
- ↑ 105.0 105.1 105.2 "Kidney-differentiated cells derived from Lowe Syndrome patient's iPSCs show ciliogenesis defects and Six2 retention at the Golgi complex". PLOS ONE 13 (2): e0192635. 2018-02-14. doi:10.1371/journal.pone.0192635. PMID 29444177. Bibcode: 2018PLoSO..1392635H.
- ↑ "Simultaneous reprogramming and gene editing of human fibroblasts". Nature Protocols 13 (5): 875–898. May 2018. doi:10.1038/nprot.2018.007. PMID 29622803.
- ↑ 107.0 107.1 107.2 107.3 "Patient-iPSC-Derived Kidney Organoids Show Functional Validation of a Ciliopathic Renal Phenotype and Reveal Underlying Pathogenetic Mechanisms". American Journal of Human Genetics 102 (5): 816–831. May 2018. doi:10.1016/j.ajhg.2018.03.014. PMID 29706353.
- ↑ 108.0 108.1 "Organoids from Nephrotic Disease-Derived iPSCs Identify Impaired NEPHRIN Localization and Slit Diaphragm Formation in Kidney Podocytes". Stem Cell Reports 11 (3): 727–740. September 2018. doi:10.1016/j.stemcr.2018.08.003. PMID 30174315.
- ↑ "Best Practices for Translational Disease Modeling Using Human iPSC-Derived Neurons". Neuron 100 (4): 783–797. November 2018. doi:10.1016/j.neuron.2018.10.033. PMID 30465765.
- ↑ "Metabolites" (in en). https://www.mdpi.com/journal/metabolites/special_issues/Intestinal_Metabolism.
- ↑ 111.0 111.1 "A functional CFTR assay using primary cystic fibrosis intestinal organoids". Nature Medicine 19 (7): 939–45. July 2013. doi:10.1038/nm.3201. PMID 23727931.
- ↑ 112.0 112.1 "Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis". Science Translational Medicine 8 (344): 344ra84. June 2016. doi:10.1126/scitranslmed.aad8278. PMID 27334259.
- ↑ "Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients". Cell Stem Cell 13 (6): 653–8. December 2013. doi:10.1016/j.stem.2013.11.002. PMID 24315439.
- ↑ "World's first mini organ transportation to a patient with ulcerative colitis" (in en). Tokyo Medical and Dental University via medicalxpress.com. https://medicalxpress.com/news/2022-08-world-mini-patient-ulcerative-colitis.html.
- ↑ Watanabe, Satoshi; Kobayashi, Sakurako; Ogasawara, Nobuhiko; Okamoto, Ryuichi; Nakamura, Tetsuya; Watanabe, Mamoru; Jensen, Kim B.; Yui, Shiro (March 2022). "Transplantation of intestinal organoids into a mouse model of colitis" (in en). Nature Protocols 17 (3): 649–671. doi:10.1038/s41596-021-00658-3. ISSN 1750-2799. PMID 35110738. https://www.nature.com/articles/s41596-021-00658-3.
- ↑ 116.0 116.1 116.2 116.3 116.4 "Modeling human development in 3D culture". Current Opinion in Cell Biology 31: 23–8. December 2014. doi:10.1016/j.ceb.2014.06.013. PMID 25033469.
- ↑ "Coordinated Morphogenetic Mechanisms Shape the Vertebrate Eye". Frontiers in Neuroscience 11: 721. 2017. doi:10.3389/fnins.2017.00721. PMID 29326547.
- ↑ Gordon, Aaron; Yoon, Se-Jin; Tran, Stephen S.; Makinson, Christopher D.; Park, Jin Young; Andersen, Jimena; Valencia, Alfredo M.; Horvath, Steve et al. (2021-02-22). "Long-term maturation of human cortical organoids matches key early postnatal transitions" (in en). Nature Neuroscience 24 (3): 331–342. doi:10.1038/s41593-021-00802-y. ISSN 1546-1726. PMID 33619405.
Further reading
- "The boom in mini stomachs, brains, breasts, kidneys and more". Nature 523 (7562): 520–2. July 2015. doi:10.1038/523520a. PMID 26223610. Bibcode: 2015Natur.523..520W.
- Kelly Rae Chi (2015). Orchestrating Organoids. A guide to crafting tissues in a dish that reprise in vivo organs. The Scientist.
- "Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation". Cell Stem Cell 16 (5): 556–65. May 2015. doi:10.1016/j.stem.2015.03.004. PMID 25891906.
- "Organoids and the genetically encoded self-assembly of embryonic stem cells". BioEssays 38 (2): 181–91. February 2016. doi:10.1002/bies.201500111. PMID 26666846.
 |
