Biology:Titin
 Generic protein structure example |

Titin[1] /ˈtaɪtɪn/ (contraction for Titan protein) (also called connectin) is a protein that in humans is encoded by the TTN gene.[2][3] Titin is a giant protein, greater than 1 µm in length,[4] that functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences.[5] These domains unfold when the protein is stretched and refold when the tension is removed.[6]
Titin is important in the contraction of striated muscle tissues. It connects the Z disc to the M line in the sarcomere. The protein contributes to force transmission at the Z disc and resting tension in the I band region.[7] It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle (cardiac or skeletal) have been correlated with differences in the mechanical properties of these muscles.[2][8]
Titin is the third most abundant protein in muscle (after myosin and actin), and an adult human contains approximately 0.5 kg of titin.[9] With its length of ~27,000 to ~35,000 amino acids (depending on the splice isoform), titin is the largest known protein.[10] Furthermore, the gene for titin contains the largest number of exons (363) discovered in any single gene,[11] as well as the longest single exon (17,106 bp).
Discovery
In 1954, Reiji Natori proposed the existence of an elastic structure in muscle fiber to account for the return to the resting state when muscles are stretched and then released.[12] In 1977, Koscak Maruyama and coworkers isolated an elastic protein from muscle fiber that they called connectin.[13] Two years later, Kuan Wang and coworkers identified a doublet band on electrophoresis gel corresponding to a high molecular weight, elastic protein that they named titin.[1][14]
In 1990, Siegfried Labeit isolated a partial cDNA clone of titin.[3] Five years later, Labeit and Bernhard Kolmerer determined the cDNA sequence of human cardiac titin.[5] In 2001, Labeit and colleagues determined the complete sequence of the human titin gene.[11][15]
Genetics
The human gene encoding for titin is located on the long arm of chromosome 2 and contains 363 exons, which together code for 38,138 amino acid residues (4200 kDa).[11] Within the gene are found a large number of PEVK (proline-glutamate-valine-lysine -abundant structural motifs) exons 84 to 99 nucleotides in length, which code for conserved 28- to 33-residue motifs that may represent structural units of the titin PEVK spring. The number of PEVK motifs in the titin gene appears to have increased during evolution, apparently modifying the genomic region responsible for titin's spring properties.[16]
Isoforms
A number of titin isoforms are produced in different striated muscle tissues as a result of alternative splicing.[17] All but one of these isoforms are in the range of ~27,000 to ~36,000 amino acid residues in length. The exception is the small cardiac novex-3 isoform, which is only 5,604 amino acid residues in length. The following table lists the known titin isoforms:
| Isoform | Alias/description | Length | Molecular weight |
|---|---|---|---|
| Q8WZ42-1 | The "canonical" sequence | 34,350 | 3,816,030 |
| Q8WZ42-2 | 34,258 | 3,805,708 | |
| Q8WZ42-3 | Small cardiac N2-B | 26,926 | 2,992,939 |
| Q8WZ42-4 | Soleus | 33,445 | 3,716,027 |
| Q8WZ42-5 | 32,900 | 3,653,085 | |
| Q8WZ42-6 | Small cardiac novex-3 | 5,604 | 631,567 |
| Q8WZ42-7 | Cardiac novex-2 | 33,615 | 3,734,648 |
| Q8WZ42-8 | Cardiac novex-1 | 34,475 | 3,829,846 |
| Q8WZ42-9 | 27,118 | 3,013,957 | |
| Q8WZ42-10 | 27,051 | 3,006,755 | |
| Q8WZ42-11 | 33,423 | 3,713,600 | |
| Q8WZ42-12 | 35,991 | 3,994,625 | |
| Q8WZ42-13 | 34,484 | 3,831,069 |
Structure
Titin is the largest known protein; its human variant consists of 34,350 amino acids, with the molecular weight of the mature "canonical" isoform of the protein being approximately 3,816,030.05 Da.[18] Its mouse homologue is even larger, comprising 35,213 amino acids with a molecular weight of 3,906,487.6 Da.[19] It has a theoretical isoelectric point of 6.02.[18] The protein's empirical chemical formula is C169,719H270,466N45,688O52,238S911.[18] It has a theoretical instability index (II) of 42.38, classifying the protein as unstable.[18] The protein's in vivo half-life, the time it takes for half of the amount of protein in a cell to break down after its synthesis in the cell, is predicted to be approximately 30 hours (in mammalian reticulocytes).[17]
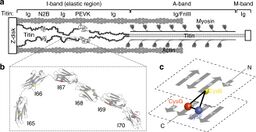
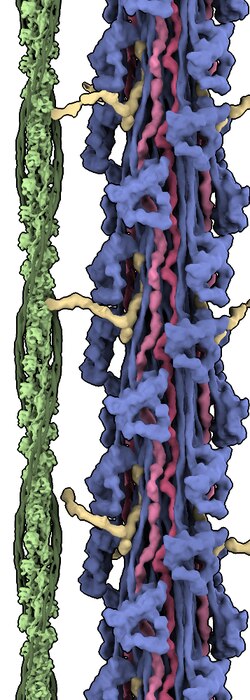
The Titin protein is located between the myosin thick filament and the Z disk.[21] Titin consists primarily of a linear array of two types of modules, also referred to as protein domains (244 copies in total): type I fibronectin type III domain (132 copies) and type II immunoglobulin domain (112 copies).[9][5] However, the exact number of these domains is different in different species. This linear array is further organized into two regions:
- N-terminal I-band: acts as the elastic part of the molecule and is composed mainly of type II modules. More specifically the I-band contains two regions of tandem type II immunoglobulin domains on either side of a PEVK region that is rich in proline (P), glutamate (E), valine (V) and lysine (K).[21]
- C-terminal A-band: is thought to act as a protein-ruler and is composed of alternating type I (Fn3) and II (Ig) modules with super-repeat segments. These have been shown to align to the 43 nm axial repeats of myosin thick filaments with immunoglobulin domains correlating to myosin crowns.[22]
The C-terminal region also contains a serine kinase domain[23][24] that is primarily known for adapting the muscle to mechanical strain.[25] It is “stretch-sensitive” and helps repair overstretching of the sarcomere.[26] The N-terminal (the Z-disc end) contains a "Z repeat" that recognizes Actinin alpha 2.[27]
The elasticity of the PEVK region has both entropic and enthalpic contributions and is characterized by a polymer persistence length and a stretch modulus.[28] At low to moderate extensions PEVK elasticity can be modeled with a standard worm-like chain (WLC) model of entropic elasticity. At high extensions PEVK stretching can be modeled with a modified WLC model that incorporates enthalpic elasticity. The difference between low-and high- stretch elasticity is due to electrostatic stiffening and hydrophobic effects.
Embedded between the PEVK and Ig residues are N2A domains.[29]
Evolution
The titin domains have evolved from a common ancestor through many gene duplication events.[30] Domain duplication was facilitated by the fact that most domains are encoded by single exons. Other giant sarcomeric proteins made out of Fn3/Ig repeats include obscurin and myomesin. Throughout evolution, titin mechanical strength appears to decrease through the loss of disulfide bonds as the organism becomes heavier.[31]
Titin A-band has homologs in invertebrates, such as twitchin (unc-22) and projectin, which also contain Ig and FNIII repeats and a protein kinase domain.[26] The gene duplication events took place independently but were from the same ancestral Ig and FNIII domains. It is said that the protein titin was the first to diverge out of the family.[24] Drosophila projectin, officially known as bent (bt), is associated with lethality by failing to escape the eggnog in some mutations as well as dominant changes in wing angles.[32][33][34]
| Titin repeat | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Titin_Ig-rpts | ||||||||
| Pfam | PF06582 | ||||||||
| InterPro | IPR010939 | ||||||||
| |||||||||
Drosophila Titin, also known as Kettin or sallimus (sls), is kinase-free. It has roles in the elasticity of both muscle and chromosomes. It is homologous to vertebrate titin I-band and contains Ig PEVK domains, the many repeats being a hot target for splicing.[35] There also exists a titin homologue, ttn-1, in C. elegans.[36] It has a kinase domain, some Ig/Fn3 repeats, and PEVT repeats that are similarly elastic.[37]
Function
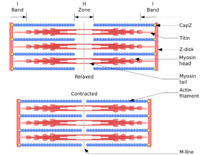
Titin is a large abundant protein of striated muscle. Titin's primary functions are to stabilize the thick filament, center it between the thin filaments, prevent overstretching of the sarcomere, and to recoil the sarcomere like a spring after it is stretched.[38] An N-terminal Z-disc region and a C-terminal M-line region bind to the Z-line and M-line of the sarcomere, respectively, so that a single titin molecule spans half the length of a sarcomere. Titin also contains binding sites for muscle-associated proteins so it serves as an adhesion template for the assembly of contractile machinery in muscle cells. It has also been identified as a structural protein for chromosomes.[39][40] Considerable variability exists in the I-band, the M-line and the Z-disc regions of titin. Variability in the I-band region contributes to the differences in elasticity of different titin isoforms and, therefore, to the differences in elasticity of different muscle types. Of the many titin variants identified, five are described with complete transcript information available.[2][3]
Dominant mutation in TTN causes predisposition to hernias.[41]
Titin interacts with many sarcomeric proteins including:[11]
- Z line region: telethonin and alpha-actinin
- I band region: calpain-3 and obscurin
- M line region: myosin-binding protein C, calmodulin 1, CAPN3, and MURF1
Clinical relevance
Mutations anywhere within the unusually long sequence of this gene can cause premature stop codons or other defects. Titin mutations are associated with hereditary myopathy with early respiratory failure,[42][43] early-onset myopathy with fatal cardiomyopathy,[44] core myopathy with heart disease, centronuclear myopathy, limb-girdle muscular dystrophy type 2J,[45] familial dilated cardiomyopathy 9,[7][46] hypertrophic cardiomyopathy and tibial muscular dystrophy.[47] Further research also suggests that no genetically linked form of any dystrophy or myopathy can be safely excluded from being caused by a mutation on the TTN gene.[45] Truncating mutations in dilated cardiomyopathy patients are most commonly found in the A region; although truncations in the upstream I region might be expected to prevent translation of the A region entirely, alternative splicing creates some transcripts that do not encounter the premature stop codon, ameliorating its effect.[48] mRNA splicing factors such as RBM20 and SLM2 (KHDRBS3) were shown to mediated alternative mRNA splicing of titin mRNA contributing to the development of heart failure due to cardiomyopathies.[49][50]
Autoantibodies to titin are produced in patients with the autoimmune disease Myasthenia gravis.[51]
Interactions
Titin has been shown to interact with:
Linguistic significance
The name titin is derived from the Greek Titan (a giant deity, anything of great size).[1]
As the largest known protein, titin also has the longest IUPAC name of a protein. The full chemical name of the human canonical form of titin, which starts methionyl... and ends ...isoleucine, contains 189,819 letters and is sometimes stated to be the longest word in the English language, or of any language.[62] However, lexicographers regard generic names of chemical compounds as verbal formulae rather than English words.[63]
References
- ↑ 1.0 1.1 1.2 "Titin: major myofibrillar components of striated muscle". Proceedings of the National Academy of Sciences of the United States of America 76 (8): 3698–3702. August 1979. doi:10.1073/pnas.76.8.3698. PMID 291034. Bibcode: 1979PNAS...76.3698W.
- ↑ 2.0 2.1 2.2 "TTN, the human gene for Titin". April 2018. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=7273.
- ↑ 3.0 3.1 3.2 "A regular pattern of two types of 100-residue motif in the sequence of titin". Nature 345 (6272): 273–276. May 1990. doi:10.1038/345273a0. PMID 2129545. Bibcode: 1990Natur.345..273L. https://ui.adsabs.harvard.edu/abs/1990Natur.345..273L/abstract. Retrieved 8 May 2022.
- ↑ "The Chain-like Elasticity of Titin". Theoretical and Computational Biophysics Group, University of Illinois. http://www.ks.uiuc.edu/Research/z1z2/.
- ↑ 5.0 5.1 5.2 "Titins: giant proteins in charge of muscle ultrastructure and elasticity". Science 270 (5234): 293–296. October 1995. doi:10.1126/science.270.5234.293. PMID 7569978. Bibcode: 1995Sci...270..293L. https://ui.adsabs.harvard.edu/abs/1995Sci...270..293L/abstract. Retrieved 8 May 2022.
- ↑ "Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils". Biophysical Journal 80 (3): 1442–1451. March 2001. doi:10.1016/S0006-3495(01)76116-4. PMID 11222304. Bibcode: 2001BpJ....80.1442M.
- ↑ 7.0 7.1 "Titin mutations as the molecular basis for dilated cardiomyopathy". Biochemical and Biophysical Research Communications 291 (2): 385–393. February 2002. doi:10.1006/bbrc.2002.6448. PMID 11846417.
- ↑ Online Mendelian Inheritance in Man (OMIM) 188840
- ↑ 9.0 9.1 "The giant protein titin. Emerging roles in physiology and pathophysiology". Circulation Research 80 (2): 290–294. February 1997. doi:10.1161/01.RES.80.2.290. PMID 9012751.
- ↑ "Damped elastic recoil of the titin spring in myofibrils of human myocardium". Proceedings of the National Academy of Sciences of the United States of America 100 (22): 12688–12693. October 2003. doi:10.1073/pnas.2133733100. PMID 14563922. Bibcode: 2003PNAS..10012688O.
- ↑ 11.0 11.1 11.2 11.3 "The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-disc to I-band Linking system". Circulation Research 89 (11): 1065–1072. November 2001. doi:10.1161/hh2301.100981. PMID 11717165.
- ↑ "Skinned Fibres of Skeletal Muscle and the Mechanism of Muscle Contraction-A Chronological Account of the Author's Investigations into Muscle Physiology". Jikeikai Medical Journal 54 (1). 1954. http://ir.jikei.ac.jp/bitstream/10328/3410/1/54-1-51.pdf. Retrieved 2014-09-09.
- ↑ "Connectin, an elastic protein of muscle. Characterization and Function". Journal of Biochemistry 82 (2): 317–337. August 1977. PMID 914784.
- ↑ "Connectin, an elastic protein of striated muscle". Biophysical Chemistry 50 (1–2): 73–85. May 1994. doi:10.1016/0301-4622(94)85021-6. PMID 8011942.
- ↑ Online Mendelian Inheritance in Man (OMIM) Titin -188840
- ↑ "Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity". Circulation Research 86 (11): 1114–1121. June 2000. doi:10.1161/01.res.86.11.1114. PMID 10850961.
- ↑ 17.0 17.1 "Titin - Homo sapiens (Human)". Universal Protein Resource. UniProt Consortium. 2010-10-05. https://www.uniprot.org/uniprot/Q8WZ42.
- ↑ 18.0 18.1 18.2 18.3 "ProtParam for human titin". ExPASy Proteomics Server. Swiss Institute of Bioinformatics. http://web.expasy.org/cgi-bin/protparam/protparam1?Q8WZ42@1-34350@.
- ↑ "ProtParam for mouse titin". ExPASy Proteomics Server. Swiss Institute of Bioinformatics. http://www.expasy.org/cgi-bin/protparam1?A2ASS6@noft@.
- ↑ "Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity". Nature Communications 9 (1): 185. January 2018. doi:10.1038/s41467-017-02528-7. PMID 29330363. Bibcode: 2018NatCo...9..185G.
- ↑ 21.0 21.1 "Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension". Proceedings of the National Academy of Sciences of the United States of America 88 (16): 7101–7105. August 1991. doi:10.1073/pnas.88.16.7101. PMID 1714586. Bibcode: 1991PNAS...88.7101W.
- ↑ "Titin domain patterns correlate with the axial disposition of myosin at the end of the thick filament". Journal of Molecular Biology 259 (5): 896–903. June 1996. doi:10.1006/jmbi.1996.0367. PMID 8683592.
- ↑ 23.0 23.1 "Structural basis for activation of the titin kinase domain during myofibrillogenesis". Nature 395 (6705): 863–869. October 1998. doi:10.1038/27603. PMID 9804419. Bibcode: 1998Natur.395..863M.
- ↑ 24.0 24.1 "The evolution of titin and related giant muscle proteins". Journal of Molecular Evolution 38 (4): 395–404. April 1994. doi:10.1007/BF00163156. PMID 8007007. Bibcode: 1994JMolE..38..395H.
- ↑ "Mechanoenzymatics of titin kinase". Proceedings of the National Academy of Sciences of the United States of America 105 (36): 13385–13390. September 2008. doi:10.1073/pnas.0805034105. PMID 18765796. Bibcode: 2008PNAS..10513385P.
- ↑ 26.0 26.1 "A Titan but not necessarily a ruler: assessing the role of titin during thick filament patterning and assembly". Anatomical Record 297 (9): 1604–1614. September 2014. doi:10.1002/ar.22987. PMID 25125174.
- ↑ "Titin, Z repeat (IPR015129) < InterPro < EMBL-EBI". http://www.ebi.ac.uk/interpro/entry/IPR015129.
- ↑ "Nature of PEVK-titin elasticity in skeletal muscle". Proceedings of the National Academy of Sciences of the United States of America 95 (14): 8052–8057. July 1998. doi:10.1073/pnas.95.14.8052. PMID 9653138. Bibcode: 1998PNAS...95.8052L.
- ↑ "Removal of immunoglobulin-like domains from titin's spring segment alters titin splicing in mouse skeletal muscle and causes myopathy". The Journal of General Physiology 143 (2): 215–230. February 2014. doi:10.1085/jgp.201311129. PMID 24470489.
- ↑ "Properties of titin immunoglobulin and fibronectin-3 domains". The Journal of Biological Chemistry 279 (45): 46351–46354. November 2004. doi:10.1074/jbc.r400023200. PMID 15322090. http://www.jbc.org/content/279/45/46351. Retrieved 2018-12-16.
- ↑ "Mechanochemical evolution of the giant muscle protein titin as inferred from resurrected proteins". Nature Structural & Molecular Biology 24 (8): 652–657. August 2017. doi:10.1038/nsmb.3426. PMID 28671667.
- ↑ "Drosophila projectin: relatedness to titin and twitchin and correlation with lethal(4) 102 CDa and bent-dominant mutants". Proceedings. Biological Sciences 249 (1324): 33–40. July 1992. doi:10.1098/rspb.1992.0080. PMID 1359548. Bibcode: 1992RSPSB.249...33F.
- ↑ "bent phenotype". http://cgslab.com/cgs1/phenotypes.cgi?n=17.
- ↑ "FlyBase Gene Report: Dmel\bt". https://flybase.org/reports/FBgn0005666.
- ↑ "D-Titin: a giant protein with dual roles in chromosomes and muscles". The Journal of Cell Biology 151 (3): 639–652. October 2000. doi:10.1083/jcb.151.3.639. PMID 11062264. PMC 2185597. http://jcb.rupress.org/content/jcb/151/3/639.full.pdf. Retrieved 2019-09-04.
- ↑ "ttn-1 (gene)". https://wormbase.org/species/c_elegans/gene/WBGene00006436.
- ↑ "Extensive and modular intrinsically disordered segments in C. elegans TTN-1 and implications in filament binding, elasticity and oblique striation". Journal of Molecular Biology 398 (5): 672–689. May 2010. doi:10.1016/j.jmb.2010.03.032. PMID 20346955.
- ↑ Anatomy & Physiology (7th ed.). McGraw Hill. 2015. p. 401. ISBN 978-0-07-340371-7.
- ↑ "Human autoantibodies reveal titin as a chromosomal protein". The Journal of Cell Biology 141 (2): 321–333. April 1998. doi:10.1083/jcb.141.2.321. PMID 9548712.
- ↑ "Titin as a Chromosomal Protein". Elastic Filaments of the Cell. Advances in Experimental Medicine and Biology. 481. 2000. pp. 221–32; discussion 232–6. doi:10.1007/978-1-4615-4267-4_13. ISBN 978-1-4613-6916-5.
- ↑ "Whole-exome sequencing identifies a potential TTN mutation in a multiplex family with inguinal hernia". Hernia 21 (1): 95–100. February 2017. doi:10.1007/s10029-016-1491-9. PMID 27115767.
- ↑ "Titin mutation segregates with hereditary myopathy with early respiratory failure". Brain 135 (Pt 6): 1695–1713. June 2012. doi:10.1093/brain/aws102. PMID 22577215.
- ↑ "Hereditary myopathy with early respiratory failure associated with a mutation in A-band titin". Brain 135 (Pt 6): 1682–1694. June 2012. doi:10.1093/brain/aws103. PMID 22577218. https://lup.lub.lu.se/search/ws/files/1591356/3900864.pdf. Retrieved 2021-09-11.
- ↑ "C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy". Annals of Neurology 61 (4): 340–351. April 2007. doi:10.1002/ana.21089. PMID 17444505.
- ↑ 45.0 45.1 "Titinopathies and extension of the M-line mutation phenotype beyond distal myopathy and LGMD2J". Neurology 64 (4): 636–642. February 2005. doi:10.1212/01.WNL.0000151853.50144.82. PMID 15728284.
- ↑ "Familial dilated cardiomyopathy locus maps to chromosome 2q31". Circulation 99 (8): 1022–1026. March 1999. doi:10.1161/01.cir.99.8.1022. PMID 10051295.
- ↑ "Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin". American Journal of Human Genetics 71 (3): 492–500. September 2002. doi:10.1086/342380. PMID 12145747.
- ↑ "HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy". Science 349 (6251): 982–986. August 2015. doi:10.1126/science.aaa5458. PMID 26315439.
- ↑ "Rbm20 regulates titin alternative splicing as a splicing repressor". Nucleic Acids Research 41 (4): 2659–2672. February 2013. doi:10.1093/nar/gks1362. PMID 23307558.
- ↑ "SLM2 Is A Novel Cardiac Splicing Factor Involved in Heart Failure due to Dilated Cardiomyopathy". Genomics, Proteomics & Bioinformatics 20 (1): 129–146. February 2022. doi:10.1016/j.gpb.2021.01.006. PMID 34273561.
- ↑ "Titin and ryanodine receptor antibodies in myasthenia gravis". Acta Neurologica Scandinavica. Supplementum 183: 19–23. 2006. doi:10.1111/j.1600-0404.2006.00608.x. PMID 16637922.
- ↑ "The hydrophilic domain of small ankyrin-1 interacts with the two N-terminal immunoglobulin domains of titin". The Journal of Biological Chemistry 278 (6): 3985–3991. February 2003. doi:10.1074/jbc.M209012200. PMID 12444090.
- ↑ 53.0 53.1 "The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules". Journal of Molecular Biology 333 (5): 951–964. November 2003. doi:10.1016/j.jmb.2003.09.012. PMID 14583192.
- ↑ "Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A". The Journal of Biological Chemistry 273 (27): 17073–17078. July 1998. doi:10.1074/jbc.273.27.17073. PMID 9642272.
- ↑ "Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence". The Journal of Biological Chemistry 270 (52): 31158–31162. December 1995. doi:10.1074/jbc.270.52.31158. PMID 8537379.
- ↑ "Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2". Journal of Cell Science 115 (Pt 24): 4925–4936. December 2002. doi:10.1242/jcs.00181. PMID 12432079.
- ↑ "Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly". The Journal of Cell Biology 154 (1): 123–136. July 2001. doi:10.1083/jcb.200102110. PMID 11448995.
- ↑ "The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity". The Journal of Cell Biology 143 (4): 1013–1027. November 1998. doi:10.1083/jcb.143.4.1013. PMID 9817758.
- ↑ "Solution scattering suggests cross-linking function of telethonin in the complex with titin". The Journal of Biological Chemistry 278 (4): 2636–2644. January 2003. doi:10.1074/jbc.M210217200. PMID 12446666.
- ↑ "Two immunoglobulin-like domains of the Z-disc portion of titin interact in a conformation-dependent way with telethonin". FEBS Letters 428 (1–2): 111–114. May 1998. doi:10.1016/S0014-5793(98)00501-8. PMID 9645487.
- ↑ "Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain". Journal of Molecular Biology 306 (4): 717–726. March 2001. doi:10.1006/jmbi.2001.4448. PMID 11243782.
- ↑ "Longest word in English". Sarah McCulloch.com. December 2009. http://www.sarahmcculloch.com/luminary-uprise/2009/longest-word/.
- ↑ Oxford Word and Language Service team. "Ask the experts - What is the longest English word?". AskOxford.com / Oxford University Press. http://www.askoxford.com/asktheexperts/faq/aboutwords/longestword.
Further reading
- "Titin: properties and family relationships". Nature Reviews. Molecular Cell Biology 4 (9): 679–689. September 2003. doi:10.1038/nrm1198. PMID 14506471.
- "Skeletal muscle-specific calpain, p49: structure and physiological function". Biochemical Pharmacology 56 (4): 415–420. August 1998. doi:10.1016/S0006-2952(98)00095-1. PMID 9763216.
- "The titin cDNA sequence and partial genomic sequences: insights into the molecular genetics, cell biology and physiology of the titin filament system". Reviews of Physiology, Biochemistry and Pharmacology 138: 19–55. 1999. doi:10.1007/BF02346659. PMID 10396137.
- "Titin: a molecular control freak". Trends in Cell Biology 9 (10): 377–380. October 1999. doi:10.1016/S0962-8924(99)01641-4. PMID 10481174.
- "Skeletal Muscle-Specific Calpain, p94, and Connectin/Titin: Their Physiological Functions and Relationship to Limb-Girdle Muscular Dystrophy Type 2A". Elastic Filaments of the Cell. Advances in Experimental Medicine and Biology. 481. 2000. pp. 383–95; discussion 395–7. doi:10.1007/978-1-4615-4267-4_23. ISBN 978-1-4613-6916-5.
- "Role of titin in vertebrate striated muscle". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 357 (1418): 199–206. February 2002. doi:10.1098/rstb.2001.1028. PMID 11911777.
- "[Titin: some aspects of the largest protein in the body]". Harefuah 141 (7): 631–5, 665. July 2002. PMID 12187564.
- "Properties of titin immunoglobulin and fibronectin-3 domains". The Journal of Biological Chemistry 279 (45): 46351–46354. November 2004. doi:10.1074/jbc.R400023200. PMID 15322090.
- "Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM". Journal of Cardiac Failure 8 (6 Suppl): S276–S286. December 2002. doi:10.1054/jcaf.2002.129278. PMID 12555133.
External links
- GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview
- GeneReviews/NCBI/NIH/UW entry on Udd Distal Myopathy, Tibial Muscular Dystrophy, Udd Myopathy
- GeneReviews/NIH/NCBI/UW entry on Salih Myopathy or Early-Onset Myopathy with Fatal Cardiomyopathy
- InterPro domain organization of titin
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |