Chemistry:1,3,5-Triheptylbenzene
From HandWiki
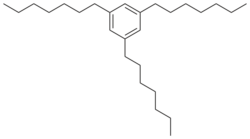
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,5-Triheptylbenzene | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C27H48 | |
| Molar mass | 372.681 g·mol−1 |
| Density | 0.855±0.06 g·cm−3[1] |
| Boiling point | 152–154 °C (425–427 K)(0.02 Torr)[2] |
| 2.3×10−8 g·L−1[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
1,3,5-Triheptylbenzene (also called sym-triheptylbenzene) is an aromatic organic compound with a chemical formula C27H48 and molar mass 372.67 g/mol.[1] It can be prepared by the hydrogenation reduction reaction of 1,1',1''-(benzene-1,3,5-triyl)tris(heptan-1-one).[2] Alternatively, 1-nonyne trimerizes to 1,3,5-triheptylbenzene when catalyzed by rhodium trichloride.[3]
References
- ↑ 1.0 1.1 1.2 Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2019 ACD/Labs). Retrieved from SciFinder. [2019-10-31]
- ↑ 2.0 2.1 Eapen, Kalathil C.; Tamborski, Christ (1988). "Synthesis of 1,3,5-tri-n-alkylbenzene compounds". The Journal of Organic Chemistry 53 (23): 5564–5567. doi:10.1021/jo00258a037. ISSN 0022-3263.
- ↑ Agenet, Nicolas; Buisine, Olivier; Slowinski, Franck; Gandon, Vincent; Aubert, Corinne; Malacria, Max (2007-03-12) (in en). Cotrimerizations of Acetylenic Compounds. John Wiley & Sons, Inc.. pp. 1–302. doi:10.1002/0471264180.or068.01. ISBN 9780471264187.
 |

