Chemistry:Ariadne (psychedelic)
| |
| Names | |
|---|---|
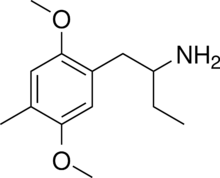
| Preferred IUPAC name
1-(2,5-Dimethoxy-4-methylphenyl)butan-2-amine | |
| Other names
4-Methyl-2,5-dimethoxy-alpha-ethylphenethylamine
4-Methyl-2,5-dimethoxybutanamine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H21NO2 | |
| Molar mass | 223.316 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ariadne (also known as 4C-D, 4C-DOM, α-Et-2C-D, BL-3912, or dimoxamine) is a lesser-known psychedelic drug. It is a homologue of 2C-D and DOM. Ariadne was first synthesized by Alexander Shulgin. In his book PiHKAL, Shulgin reported testing Ariadne up to a dose of 32 mg, and reported that it produces psychedelia at a bare threshold.[1] Very little data exists about the pharmacological properties, metabolism, and toxicity of Ariadne in humans apart from Shulgin's limited testing.
Shulgin reported that the drug was tested by Bristol Laboratories as an antidepressant, in an anecdote where he was explaining how human testing is invaluable (compared to animal testing) on drugs that change the state of the mind. He said, "Before they launched into a full multi-clinic study to determine if it's going to be worth the animal studies or not, every person on the board of directors took it."[2]
In an animal study, Ariadne was shown to produce stimulus generalization in rats trained to respond to the drug MDMA.[3]
See also
- 4C-B
- 4C-T-2
- Phenethylamine
- Psychedelics, dissociatives and deliriants
References
- ↑ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. http://www.erowid.org/library/books_online/pihkal/pihkal.shtml.
- ↑ Alexander Shulgin (2021). The Nature of Drugs. Berkeley, California: Transform Press. pp. 299–300. ISBN 9780999547212.
- ↑ Glennon RA (1993). "MDMA-like stimulus effects of alpha-ethyltryptamine and the alpha-ethyl homolog of DOM". Pharmacol Biochem Behav 46 (2): 459–462. doi:10.1016/0091-3057(93)90379-8. PMID 7903460.

