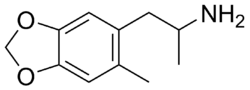
Chemistry:6-Methyl-MDA
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H15NO2 |
| Molar mass | 193.246 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
6-Methyl-3,4-methylenedioxyamphetamine (6-Methyl-MDA) is an entactogen and psychedelic drug of the amphetamine class.[1] It was first synthesized in the late 1990s by a team including David E. Nichols at Purdue University while investigating derivatives of 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxy-N-methylamphetamine (MDMA).[1]
6-Methyl-MDA has IC50 values of 783 nM, 28,300 nM, and 4,602 nM for inhibiting the reuptake of serotonin, dopamine, and norepinephrine in rat synaptosomes.[1] In animal studies it substitutes for MBDB, MMAI, LSD, and 2,5-dimethoxy-4-iodoamphetamine (DOI), though not amphetamine, but only partially and at high doses.[1] Thus, while several-fold less potent than its analogues 2-methyl-MDA and 5-methyl-MDA, and approximately half as potent as MDA, 6-methyl-MDA is still significantly active,[1] and appropriate doses may be similar to or somewhat higher than those of MDMA.[citation needed]
References
 |

