Biology:5-IT
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
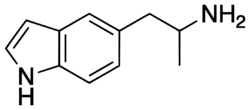
| Formula | C11H14N2 |
| Molar mass | 174.247 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
5-(2-Aminopropyl)indole (5-API, 5-IT, PAL-571)[1] is an indole and phenethylamine derivative with empathogenic effects. Its preparation was first reported by Albert Hofmann in 1962.[2] It is a designer drug that has been openly sold as a recreational drug by online vendors since 2011.[3]
Chemistry
Although 5-IT is a positional isomer of the tryptamine drug αMT, the compound is not itself a tryptamine as the indole ring is substituted at the 5 position rather than at the 3 position. The compound is closer chemically to phenethylamine derivatives such as 5-APB. This is reflected in the compound's effects when used as a drug, which are reportedly stimulating rather than psychedelic.
Pharmacology
5-IT acts as a triple monoamine releasing agent with EC50 values of 12.9 nM for dopamine, 13.3 nM for norepinephrine and 104.8 nM for serotonin and also as MAO-A inhibitor.[4][5]
Dosage and effects
Alexander Shulgin wrote briefly about 5-IT in TiHKAL saying: "at 20 milligrams orally, [it] is a long-lived stimulant producing increased heart-rate, anorexia, diuresis, and slight hyperthermia for about twelve hours."[6] As 5-IT is not a tryptamine and thus not within the scope of the book, it is not discussed in any more detail than this.
The following symptoms can indicate 5-IT has been ingested: hyperthermia, tachycardia, increased blood pressure, dilated pupils (mydriasis), agitation, excessive sweating, jaw clenching, insomnia, disorientation, restlessness, anxiety, and tremor.[3] It is a MAOI, and when combined with a contraindicated substance, it can lead to death.
Deaths
5-IT has been attributed to 14 deaths of people in Sweden since its discovery.[7][8] 5-IT was listed as the sole intoxicant in two cases but other drugs were also found in the twelve other post mortem examinations. The 14 deaths occurred between April and July 2012, but a definitive identification of 5-IT in the post-mortem samples was not made until July. All of the dead were young men aged between 20 and 30. Eleven non-fatal poisonings due to 5-IT also reportedly occurred during the same time period.[9]
Legality
- 5-IT is a positional isomer of αMT, and as such is considered legally the same as αMT under the Controlled Substance Act in the US. (The Federal Analog Act includes a clause concerning the effects of the substance as well.)
- 5-IT was banned as a temporary class drug in June 2013, along with 9 other related compounds.[10] On March 5, 2014, the UK Home Office announced that 5-API would be made a class B drug on 10 June 2014 alongside every other benzofuran entactogen and many structurally related drugs.[11]
- 5-IT is covered by the Australian analogue act as an analogue of MDA "by the replacement of up to 2 carbocyclic or heterocyclic ring structures with different carbocyclic or heterocyclic ring structures".[12]
- A formal application for 5-IT to be made illegal in Sweden was made on July 26, 2012, but did not come into effect immediately.
- 5-IT was made illegal in Denmark on 30 September 2012.
- 5-IT is an Anlage I controlled drug in Germany.
- The European Commission published a proposal for a decision calling upon its member states to take measures to control 5-(2-aminopropyl)indole. It asked member states to introduce control measures and criminal penalties as provided under their national legislation covering psychotropic substances.[13]
See also
- 6-IT
- 6-APB
- αMT
References
- ↑ "Abuse-related effects of dual dopamine/serotonin releasers with varying potency to release norepinephrine in male rats and rhesus monkeys". Experimental and Clinical Psychopharmacology 22 (3): 274–284. June 2014. doi:10.1037/a0036595. PMID 24796848.
- ↑ Hofmann, Albert; Troxler, Franz, "Nouveaux derives de l'indole et leur preparation", FR patent 1344579
- ↑ 3.0 3.1 "5-(2-aminopropyl)indole: a new player in the drama of 'legal highs' alerts the community". Drug and Alcohol Review 34 (1): 51–7. January 2015. doi:10.1111/dar.12136. PMID 24634984.
- ↑ "The new psychoactive substances 5-(2-aminopropyl)indole (5-IT) and 6-(2-aminopropyl)indole (6-IT) interact with monoamine transporters in brain tissue". Neuropharmacology 101: 68–75. February 2016. doi:10.1016/j.neuropharm.2015.09.004. PMID 26362361.
- ↑ "5-(2-Aminopropyl)indole (5-IT): a psychoactive substance used for recreational purposes is an inhibitor of human monoamine oxidase (MAO)". Drug Testing and Analysis 6 (7–8): 607–13. July–August 2014. doi:10.1002/dta.1530. PMID 24115740.
- ↑ Shulgin, Alexander (December 1997). Tihkal: A Continuation [Paperback]. Transform Press. ISBN 978-0-9630096-9-2. https://www.erowid.org/library/books_online/tihkal/tihkal48.shtml. Retrieved 2012-02-08.
- ↑ "Nätdrog dödade 14 unga män" (in Swedish). Aftonbladet. 28 July 2012. http://www.aftonbladet.se/nyheter/article15178324.ab.
- ↑ "Deaths associated with new designer drug 5-IT". BMJ 345: e5625. August 2012. doi:10.1136/bmj.e5625. PMID 22923530. http://eprints.hud.ac.uk/id/eprint/18545/1/Maskellbmj.e5625.full.pdf.
- ↑ "Fem nya ämnen klassas som narkotika" (in Swedish). The Swedish National Institute of Public Health. http://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2014/augusti/fem-nya-amnen-klassas-som-narkotika/.
- ↑ "Temporary class drug order report on 5-6APB and NBOMe compounds". UK Home Office. 4 Jun 2013. https://www.gov.uk/government/publications/temporary-class-drug-order-report-on-benzofury-and-nbome-compounds.
- ↑ UK Home Office (2014-03-05). "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Government. http://www.legislation.gov.uk/ukdsi/2014/9780111110904.
- ↑ "Criminal Code Act 1995". Australian Government. 2009-08-05. http://www.comlaw.gov.au/ComLaw/Legislation/ActCompilation1.nsf/0/1B4A2DD73EF9A4BBCA2576040024B600/$file/CriminalCode1995_WD02.pdf. "PAGE 503"
- ↑ "COM(2013) 436 final" (PDF). European Commission. 2013-06-25. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2013:0436:FIN:EN:PDF.
