Chemistry:6-APT
From HandWiki
Short description: Chemical compound
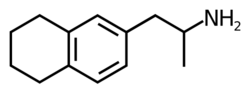 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H19N |
| Molar mass | 189.302 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
6-(2-Aminopropyl)tetralin (6-APT), also sometimes called tetralinylaminopropane (TAP), is a drug of the amphetamine class which acts as a selective serotonin releasing agent (SSRA).[1] It has IC50 values of 121 nM, 6,436 nM, and 3,371 nM for inhibiting the reuptake of serotonin, dopamine, and norepinephrine, respectively.[1] Though it possesses an appreciable in vitro profile, in animal drug discrimination studies it was not found to substitute for MMAI or amphetamine and to only partially substitute for MBDB.[1] This parallels Alexander Shulgin's finding that EDMA (the 1,4-benzodioxine analogue of 6-APT) is inactive,[2] and appears to indicate that the pharmacokinetics of both EDMA and 6-APT may not be favorable.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 "Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine". Journal of Medicinal Chemistry 36 (23): 3700–3706. November 1993. doi:10.1021/jm00075a027. PMID 8246240.
- ↑ "EDMA · 3,4-Ethylenedioxy-N-methylamphetamine". Pihkal: A Chemical Love Story. Transform Press. 13 May 2016. ISBN 978-0-9630096-0-9. http://isomerdesign.com/PiHKAL/read.php?domain=pk&id=110.
 |
