Chemistry:Axillarin
From HandWiki
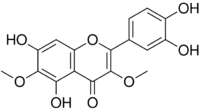
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3,6-dimethoxy-4H-1-benzopyran-4-one | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H14O8 | |
| Molar mass | 346.291 g·mol−1 |
| Density | 1.659 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Axillarin is an O-methylated flavonol. It can be found in Pulicaria crispa, Filifolium sibiricum, Inula britannica,[1] Wyethia bolanderi in Balsamorhiza macrophylla[2] and in Tanacetum vulgare.[3] It can also be synthesized.[4]
Glycosides
Axillarin 7-O-β-D-glucoside can be found in Tagetes mendocina, a plant used in traditional herbal medicine the Andean provinces of Argentina.[5]
References
- ↑ Jung Park, Eun; Kim, Youngleem; Kim, Jinwoong (2000). "Acylated Flavonol Glycosides from the Flower ofInula britannica". Journal of Natural Products 63 (1): 34–36. doi:10.1021/np990271r. PMID 10650074.
- ↑ McCormick, Susan; Robson, Kathleen; Bohm, Bruce (1985). "Methylated flavonols from Wyethia bolanderi and Balsamorhiza macrophylla". Phytochemistry 24 (9): 2133. doi:10.1016/S0031-9422(00)83143-X.
- ↑ Álvarez, Ángel L.; Habtemariam Solomon; Juan-Badaturuge Malindra; Jackson Caroline; Parra Francisco (2011). "In vitro anti HSV-1 and HSV-2 activity of Tanacetum vulgare extracts and isolated compounds: An approach to their mechanisms of action". Phytotherapy Research 25 (2): 296–301. doi:10.1002/ptr.3382. PMID 21171142. https://hal.archives-ouvertes.fr/hal-00601639.
- ↑ Fukui, K.; Nakayama, M.; Horie, T. (1968). "The syntheses of axillarin and its related compounds". Experientia 24 (8): 769–770. doi:10.1007/BF02144856.
- ↑ Guillermo Schmeda-Hirschmann, Alejandro Tapia, Cristina Theoduloz, Jaime Rodrıguez, Susana Lopez and Gabriela Egly Feresin (2004). "Free Radical Scavengers and Antioxidants from Tagetes mendocina". Verlag der Zeitschrift für Naturforschung 59c: 345–353. http://www.znaturforsch.com/ac/v59c/s59c0345.pdf.
 |
