Chemistry:Jaceidin
From HandWiki
| |
| Names | |
|---|---|
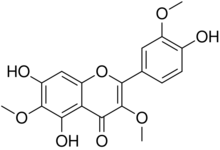
| IUPAC name
4′,5,7-Trihydroxy-3,3′,6-trimethoxyflavone
| |
| Systematic IUPAC name
5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,6-dimethoxy-4H-1-benzopyran-4-one | |
| Other names
Jaceidine
Quercetagetin 3,3′,6-trimethyl ether | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C18H16O8 | |
| Molar mass | 360.318 g·mol−1 |
| Melting point | 130–135 °C (266–275 °F; 403–408 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Jaceidin is an O-methylated flavonol. It can be found in Chamomilla recutita,[1] in Centaurea jacea and can be synthesized.[2] Jaceidin has many different characteristics, such as a molar mass of 360.31 g/mol. It also has a melting point of 130-135 °C.[3]
Glycosides
- Jacein
References
- ↑ Repčák, Miroslav; Švehlı́Ková, Vanda; Imrich, Ján; Pihlaja, Kalevi (1999). "Jaceidin and chrysosplenetin chemotypes of Chamomilla recutita (L.) Rauschert". Biochemical Systematics and Ecology 27 (7): 727–732. doi:10.1016/S0305-1978(98)00124-0.
- ↑ Fukui, K.; Matsumoto, T.; Nakamura, S.; Nakayama, M.; Horie, T. (1968). "The synthesis of jaceidin". Experientia 24 (2): 108–109. doi:10.1007/BF02146923. PMID 5643784.
- ↑ "Jaceidin". Human Metabolome Database. http://www.hmdb.ca/metabolites/HMDB0033819.
 |

