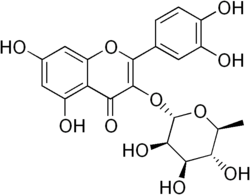
Chemistry:Quercitrin
| |
| |
| Names | |
|---|---|
| IUPAC name
3′,4′,5,7-Tetrahydroxy-3-(α-L-rhamnopyranosyloxy)flavone
| |
| Systematic IUPAC name
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Quercetin 3-O-a-L-rhamnoside
Thujin Quercetin 3-rhamnoside Quercetin-3-rhamnoside Quercetin-3-L-rhamnoside | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H20O11 | |
| Molar mass | 448.38 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Quercitrin is a glycoside formed from the flavonoid quercetin and the deoxy sugar rhamnose.
Austrian chemist Heinrich Hlasiwetz (1825-1875) is remembered for his chemical analysis of quercitrin.
Occurrence
Quercitrin is a constituent of the dye quercitron. It can be found in Tartary buckwheat (Fagopyrum tataricum)[1] and in oaks species like the North American white oak (Quercus alba) and English oak (Quercus robur).[2] It is also found in Nymphaea odorata or Taxillus kaempferi.[3]
Metabolism
The enzyme quercitrinase catalyzes the chemical reaction between quercitrin and H2O to yield L-rhamnose and quercetin.
References
- ↑ Tartary Buckwheat (Fagopyrum tataricum Gaertn.) as a Source of Dietary Rutin and Quercitrin. Nina Fabjan, Janko Rode, Iztok Jože Košir, Zhuanhua Wang, Zheng Zhang and Ivan Kreft, J. Agric. Food Chem., 2003, 51 (22), pp. 6452–6455, doi:10.1021/jf034543e
- ↑ Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry. Pirjo Mämmelä, Heikki Savolainen, Lasse Lindroos, Juhani Kangas and Terttu Vartiainen, Journal of Chromatography A, Volume 891, Issue 1, 1 September 2000, Pages 75-83, doi:10.1016/S0021-9673(00)00624-5
- ↑ The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of Taxillus kaempferi. Konishi T, Nishio T, Kiyosawa S, Fujiwara Y and Konoshima T, Yakugaku Zasshi., February 1996, volume 116, issue 2, pages 148-157 (article in Japanese), doi:10.1248/yakushi1947.116.2_148
Audah, K.A.; Ettin, J.; Darmadi, J.; Azizah, N.N.; Anisa, A.S.; Hermawan, T.D.F.; Tjampakasari, C.R.; Heryanto, R.; Ismail, I.S.; Batubara, I. Indonesian Mangrove Sonneratia caseolaris Leaves Ethanol Extract Is a Potential Super Antioxidant and Anti Methicillin-Resistant Staphylococcus aureus Drug. Molecules 2022, 27, 8369. https://doi.org/10.3390/molecules27238369
 |
