Chemistry:Isoquercetin
| |
| Names | |
|---|---|
| IUPAC name
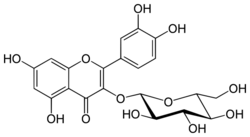
3-(β-D-Glucopyranosyloxy)-3′,4′,5,7-tetrahydroxyflavone
| |
| Systematic IUPAC name
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Isoquercitroside
Isoquercitin Isoquercetin Trifoliin Isotrifolin Trifoliin A Isohyperoside Isotrifoliin Quercetin-3-glucoside Quercetin-3-O-glucoside Quercetin 3-O-β-D-glucopyranoside | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H20O12 | |
| Molar mass | 464.379 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isoquercetin, isoquercitrin or isotrifoliin[1] is a flavonoid, a type of chemical compound. It is the 3-O-glucoside of quercetin. Isoquercitrin can be isolated from various plant species including Mangifera indica (mango)[2] and Rheum nobile (the Noble rhubarb). It is also present in the leaves of Annona squamosa, Camellia sinensis (tea).[3][4] and Vestia foetida[5]
Spectral data
The lambda-max for isoquercetin is 254.8 and 352.6 nm.
Potential clinical uses
Isoquercetin is presently being investigated for prevention of thromboembolism in selected cancer patients[6] and as an anti-fatigue agent in kidney cancer patients treated with sunitinib.[7]
There is a single case report of its use in the successful treatment of prurigo nodularis, a difficult to treat pruritic eruption of the skin.[8]
However it belongs to the PAINS (Pan-assay interference compounds) categories of chemicals.[9]
References
- ↑ "Isoquercetin". PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5280804#section=Top.
- ↑ "Characterization of phenolic compounds in some Indian mango cultivars". International Journal of Food Sciences and Nutrition 55 (2): 163–9. 2004. doi:10.1080/09637480410001666441. PMID 14985189.
- ↑ Panda S, Kar A (2007). "Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside". BioFactors 31 (3-4): 201–210. doi:10.1002/biof.5520310307. PMID 18997283.
- ↑ Sakakibara, H; Honda, Y; Nakagawa, S; Ashida, H; Kanazawa, K (2003). "Simultaneous determination of all polyphenols in vegetables, fruits, and teas". Journal of Agricultural and Food Chemistry 51 (3): 571–81. doi:10.1021/jf020926l. PMID 12537425.
- ↑ C. Brevis, M. Quezada, P. Bustamante, L. Carrasco, A. Ruiz, S. Donoso, Huevil (Vestia foetida) poisoning of cattle in Chile The Veterinary record 156(14):452-3 May 2005
- ↑ NCT02195232
- ↑ NCT02446795
- ↑ Pennesi, Christine M.; Neely, John; Marks Jr., Ames G.; Alison Basak, S. (November 2017). "Use of Isoquercetin in the Treatment of Prurigo Nodularis". Journal of Drugs in Dermatology 16 (11): 1156–1158. http://jddonline.com/articles/dermatology/S1545961617P1156X/1.
- ↑ J Baell; M A Walters (2014). "Chemistry: Chemical con artists foil drug discovery". Nature 513 (7519): 481–483. doi:10.1038/513481a. PMID 25254460.
See also
 |
