Chemistry:Isorhamnetin
| |

| |
| Names | |
|---|---|
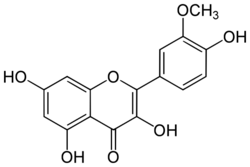
| IUPAC name
3,4′,5,7-Tetrahydroxy-3′-methoxyflavone
| |
| Systematic IUPAC name
3,5,7-Trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
3-methylquercetin
3-Methylquercetin Isorhamnetol isorhamentin isorhamnetine iso-rhamnetin 3'-Methoxyquercetin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H12O7 | |
| Molar mass | 316.26 g/mol |
| Melting point | 307 °C (585 °F; 580 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isorhamnetin is an O-methylated flavon-ol from the class of flavonoids. A common food source of this 3'-methoxylated derivative of quercetin and its glucoside conjugates are pungent yellow or red onions, in which it is a minor pigment, quercetin-3,4'-diglucoside and quercetin-4'-glucoside and the aglycone quercetin being the major pigments.[1] Pears, olive oil, wine and tomato sauce are rich in isorhamnetin.[2] Almond skin is a rich source of isorhamnetin-3-O-rutinoside and isorhamnetin-3-O-glucoside, in some cultivars they comprise 75% of the polyphenol content, the total of which can exceed 10 mg/100 gram almond.[3] Others sources include the spice, herbal medicinal[4] and psychoactive Mexican tarragon (Tagetes lucida), which is described as accumulating isorhamnetin and its 7-O-glucoside derivate.[5] Nopal (Opuntia ficus-indica (L.)) is also a good source of isorhamnetin, which can be extracted by supercritical fluid extraction assisted by enzymes.[6]
Metabolism
The enzyme quercetin 3-O-methyltransferase uses S-adenosyl methionine and quercetin to produce S-adenosylhomocysteine and isorhamnetin.
The enzyme 3-methylquercetin 7-O-methyltransferase uses S-adenosyl methionine and 5,7,3',4'-tetrahydroxy-3-methoxyflavone (isorhamnetin) to produce S-adenosylhomocysteine and 5,3',4'-trihydroxy-3,7-dimethoxyflavone (rhamnazin).
Glycosides
- Isorhamnetin-3-O-rutinoside-7-O-glucoside
- Isorhamnetin-3-O-rutinoside-4'-O-glucoside
- Narcissin (Isorhamnetin-3-O-rutinoside)
See also
- List of antioxidants in food
- List of phytochemicals in food
- Tamarixetin, the 4'-methyl analog
References
- ↑ Slimestad, R; Fossen, T; Vågen, IM (December 2007). "Onions: a source of unique dietary flavonoids". J. Agric. Food Chem. 55 (25): 10067–80. doi:10.1021/jf0712503. PMID 17997520.
- ↑ Holland, Thomas M.; Agarwal, Puja; Wang, Yamin; Leurgans, Sue E.; Bennett, David A.; Booth, Sarah L.; Morris, Martha Clare (2020-01-29). "Dietary flavonols and risk of Alzheimer dementia" (in en). Neurology 94 (16): e1749–e1756. doi:10.1212/WNL.0000000000008981. ISSN 0028-3878. PMID 31996451.
- ↑ PMID 25544797 PMC4276397
- ↑ Céspedes, Carlos L. (2006). "Antifungal and Antibacterial Activities of Mexican Tarragon (Tagetes lucida)". Journal of Agricultural and Food Chemistry 54 (10): 3521–3527. doi:10.1021/jf053071w. PMID 19127719.
- ↑ Abdala, 1999
- ↑ "Supercritical CO2 enzyme hydrolysis as a pretreatment for the release of isorhamnetin conjugates from Opuntia ficus-indica (L.) Mill". The Journal of Supercritical Fluids 141: 21–28. November 2018. doi:10.1016/j.supflu.2017.11.030.
External links
 |
