Chemistry:Barium sulfite
From HandWiki
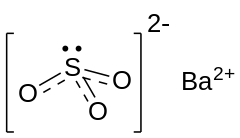
| |
| Names | |
|---|---|
| IUPAC name
Barium sulfite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| BaSO3 | |
| Molar mass | 217.391 g/mol |
| Appearance | white monoclinic crystals |
| Density | 4.44 g/cm3 |
| Melting point | decomposes |
| 0.0011 g/100 mL | |
| Solubility | insoluble in ethanol[1] |
| Related compounds | |
Other anions
|
Barium sulfate Barium fluoride Barium chloride Barium bromide Barium iodide |
Other cations
|
Calcium sulfite Magnesium sulfite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Barium sulfite is the inorganic compound with the chemical formula BaSO3. It is a white powder that finds few applications. It is an intermediate in the carbothermal reduction of barium sulfate to barium sulfide:[2]
- BaSO4 + CO → BaSO3 + CO2
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), CRC Press, pp. 4–45, ISBN 0-8493-0594-2
- ↑ Kresse, Robert; Baudis, Ulrich; Jäger, Paul; Riechers, H. Hermann; Wagner, Heinz; Winkler, Jochen; Wolf, Hans Uwe (2007). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_325.pub2.
 |
