Chemistry:Barium chlorate
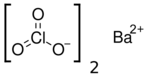
| |

| |
| Names | |
|---|---|
| IUPAC name
Barium dichlorate
| |
| Other names
Chloric acid, barium salt
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1445 |
| |
| |
| Properties | |
| Ba(ClO3)2 | |
| Molar mass | 304.23 g/mol |
| Appearance | white solid |
| Density | 3.18 g/cm3, solid |
| Melting point | 413.9 °C (777.0 °F; 687.0 K) (decomposes) |
| 27.5 g/100 ml (20 °C) | |
| −87.5·10−6 cm3/mol | |
| Hazards[1] | |
| Safety data sheet | Barium Chlorate MSDS |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H271, H302, H332, H411 | |
| P210, P220, P221, P261, P264, P270, P271, P273, P280, P283, P301+312, P304+312, P304+340, P306+360, P312, P330, P370+378, P371+380+375, P391, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
500.1 mg/kg |
LC50 (median concentration)
|
(4h) 1.5 mg/l - dust/mist |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
0.5 mg/m3 (Vacated) |
IDLH (Immediate danger)
|
50 mg/m3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Barium chlorate, Ba(ClO3)2, is the barium salt of chloric acid. It is a white crystalline solid, and like all soluble barium compounds, irritant and toxic. It is sometimes used in pyrotechnics to produce a green colour. It also finds use in the production of chloric acid.
Reactions
Synthesis
Barium chlorate can be produced through a double replacement reaction between solutions of barium chloride and sodium chlorate:
- BaCl
2 + 2 NaClO
3 → Ba(ClO
3)
2 + 2 NaCl
After concentrating and cooling the resulting mixture, barium chlorate precipitates. This is perhaps the most common preparation, exploiting the lower solubility of barium chlorate compared to sodium chlorate.[citation needed]
The above method does result in some sodium contamination, which is undesirable for pyrotechnic purposes, where the strong yellow colour of sodium can easily overpower the green of barium. Sodium-free barium chlorate can be produced directly through electrolysis:[2]
- BaCl
2 + 6 H
2O → Ba(ClO
3)
2 + 6 H
2
It can also be produced by the reaction of barium carbonate with boiling ammonium chlorate solution:[3]: 314–315
- 2 NH
4ClO
3 + BaCO
3 → Ba(ClO
3)
2 + 2 NH
3 + H
2O + CO
2
The reaction initially produces barium chlorate and ammonium carbonate; boiling the solution decomposes the ammonium carbonate and drives off the resulting ammonia and carbon dioxide, leaving only barium chlorate in solution.

Decomposition
When exposed to heat, barium chlorate alone will decompose to barium chloride and oxygen:
- Ba(ClO
3)
2 → BaCl
2 + 3 O
2
Chloric acid
Barium chlorate is sometimes used to produce chloric acid.[3]: 312–313
Commercial uses
When barium chlorate is heated with a fuel, it burns to produce a vibrant green light, which is also a flame test for the presence of bariom ions. Because it is an oxidizer, a chlorine donor, and contains a metal ion, this compound produces a distinctive green colour. However, due to the instability of all chlorates to sulfur, acids, and ammonium ions, chlorates have been banned from use in class C fireworks in the United States. Therefore, more and more firework producers have begun to use more stable compounds such as barium nitrate and barium carbonate.[4]
Toxicity
Barium chlorate is dangerous to human health, causing severe acute effects after high exposure. At lower levels, it is irritating to the skin, nasal passages, and throat, and can cause nausea, vomiting, diarrhea, and abdominal pain. At high levels it may cause methemoglobinemia, a condition where the blood can no longer carry sufficient oxygen. This results in a range of effects from dizziness and lightheadedness to trouble breathing, collapse, and death depending on exposure level. It may also cause tremors, seizures, muscle twitching, and irregular heartbeat.[5]
As a soluble heavy metal salt it has the potential to cause heavy metal poisoning and effects such as kidney damage from long term low level exposures that do not produce immediate symptoms. It may also cause bright spots in the lungs in chest x-rays, a benign condition known as baritosis.[5]
Environmental Hazard
It is very harmful to aquatic organisms if it is leached into bodies of water.[6] It may be necessary to dispose of this compound as hazardous waste, depending on local and or federal laws.[5]
References
- ↑ Sigma-Aldrich Co., Barium chlorate. Retrieved on 6 December 2024.
- ↑ Perigrin, Tom. "Barium Chlorate". GeoCities. Archived from the original on 2007-10-30. https://web.archive.org/web/20071030002126/http://www.geocities.com/CapeCanaveral/Campus/5361/chlorate/barium.html. Retrieved 2007-02-22.
- ↑ 3.0 3.1 Brauer, Georg; Schmeisser, M. (1963). "5. Chlorine, Bromine, Iodine". in Riley, Reed F.. Handbook of Preparative Inorganic Chemistry (2nd ed.). New York, London: Academic Press. pp. 314-315. ISBN 9780121266011. https://archive.org/details/Handbook_of_Preparative_Inorganic_Chemistry_1_2_Brauer/page/n337/mode/2up. Retrieved 6 December 2024.
- ↑ Wilson, Elizabeth (July 2, 2001). "What's That Stuff? Fireworks". Chemical & Engineering News 79 (27): 30. https://pubsapp.acs.org/cen/whatstuff/stuff/7927sci3.html.
- ↑ 5.0 5.1 5.2 "Barium Chlorate". New Jersey Department of Health and Human Services. August 2001. http://nj.gov/health/eoh/rtkweb/documents/fs/0183.pdf.
- ↑ "ICSC 0613 - Barium Chlorate". ILO & WHO: INCHEM. 2021. http://www.inchem.org/documents/icsc/icsc/eics0613.htm.
 |

