Chemistry:Barium bromide
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| BaBr2 (anhydrous) BaBr2·2H2O (dihydrate) | |
| Molar mass | 297.14 g/mol |
| Appearance | White solid |
| Density | 4.78 g/cm3 (anhydrous) 3.58 g/cm3 (dihydrate) |
| Melting point | 857 °C (1,575 °F; 1,130 K) |
| Boiling point | 1,835 °C (3,335 °F; 2,108 K) |
| 92.2 g/100 mL (0°C) | |
| -92.0·10−6 cm3/mol | |
| Structure | |
| PbCl2-type (orthorhombic, oP12) | |
| Pnma (No. 62) | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−181.1 kcal/mol |
| Hazards | |
| Main hazards | Toxic |
| Safety data sheet | NIH BaBr |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H332 | |
| P261, P264, P270, P271, P301+312, P304+312, P304+340, P312, P330, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Barium fluoride Barium chloride Barium iodide |
Other cations
|
Beryllium bromide Magnesium bromide Calcium bromide Strontium bromide Radium bromide Lead bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
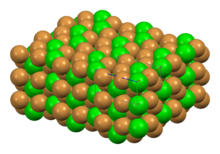
Barium bromide is the chemical compound with the formula BaBr2. It is ionic and hygroscopic in nature.
Structure and properties
BaBr2 crystallizes in the lead chloride (cotunnite) motif, giving white orthorhombic crystals that are deliquescent.[1][2]
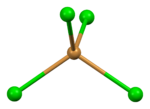
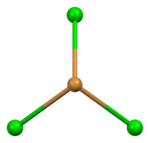
| Ion | Ba2+ | Br− (tetrahedral) | Br− (trigonal) |
|---|---|---|---|
| Coordination sphere | {BaBr9} | {BrBa4} | {BrBa3} |
| Ball-and-stick model | 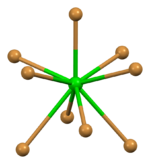
|
|
|
| Coordination number | 9 | 4 | 3 |
| Coordination geometry | (7+2) coordination[5] distorted tricapped trigonal prismatic |
distorted tetrahedral | trigonal pyramidal |
In aqueous solution BaBr2 behaves as a simple salt.
Solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate.
- BaBr2 + SO2−4 → BaSO4 + 2 Br−
Similar reactions occur with oxalic acid, hydrofluoric acid, and phosphoric acid, giving solid precipitates of barium oxalate, fluoride, and phosphate, respectively.
Preparation
Barium bromide can be prepared by treating barium sulfide or barium carbonate with hydrobromic acid:
- BaS + 2 HBr → BaBr2 + H2S
- BaCO3 + 2 HBr → BaBr2 + CO2 + H2O
Barium bromide crystallizes from concentrated aqueous solution in its dihydrate , BaBr2·2H2O. Heating this dihydrate to 120 °C gives the anhydrous salt. [6]
Uses
Barium bromide is a precursor to chemicals used in photography and to other bromides.
Historically, barium bromide was used to purify radium in a process of fractional crystallization devised by Marie Curie. Since radium precipitates preferentially in a solution of barium bromide, the ratio of radium to barium in the precipitate would be higher than the ratio in the solution.[7]
Safety
Barium bromide, along with other water-soluble barium salts (e.g. barium chloride), is toxic. However, there is no conclusive data available on its hazards.[8]
In popular culture
The compound appears in the intro title card of Breaking Bad, where the first pairs of letters are replaced with Br35 and Ba56, the symbols and atomic numbers of bromine and barium respectively.
References
- ↑ 1.0 1.1 Brackett, Elizabeth B.; Brackett, Thomas E.; Sass, Ronald L. (1963). "The Crystal Structures of Barium Chloride, Barium Bromide, and Barium Iodide". J. Phys. Chem. 67 (10): 2132–2135. doi:10.1021/j100804a038.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 117-119. ISBN 978-0-08-037941-8.
- ↑ "Information card for entry 1527183". 1963. http://www.crystallography.net/cod/1527183.html.
- ↑ "ICSD 15706 : ICSD Structure : Ba Br2". Cambridge Crystallographic Data Centre. https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=1596411&DatabaseToSearch=Published.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 382. ISBN 978-0-08-037941-8.
- ↑ Patnaik, Pradyot (2003), Handbook of Inorganic Chemical Compounds, McGraw-Hill Professional, pp. 81–82, ISBN 978-0-07-049439-8, https://books.google.com/books?id=Xqj-TTzkvTEC&q=%22barium+bromide%22+subject:%22Chemistry,+Inorganic%22&pg=RA1-PA81, retrieved 2007-12-03
- ↑ Sime, Ruth Lewin (1996), Lise Meitner: A Life in Physics, University of California Press, p. 233, ISBN 978-0-520-20860-5, https://books.google.com/books?id=uPzZQzx-mkcC&q=%22barium+bromide%22+radium&pg=PA233, retrieved 2007-12-03
- ↑ https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=413607&brand=ALDRICH |
 |

