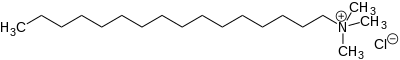
Chemistry:Cetrimonium chloride
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N-Trimethylhexadecan-1-aminium chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H42ClN | |
| Molar mass | 320.00 g/mol |
| Pharmacology | |
| 1=ATC code }} | D08AJ02 (WHO) R02AA17 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Cetrimonium chloride, or cetyltrimethylammonium chloride (CTAC), is a topical antiseptic and surfactant. Long-chain quaternary ammonium surfactants, such as cetyltrimethylammonium chloride (CTAC), are generally combined with long-chain fatty alcohols, such as stearyl alcohols, in formulations of hair conditioners and shampoos.[1][2] The cationic surfactant concentration in conditioners is generally of the order of 1–2% and the alcohol concentrations are usually equal to or greater than those of the cationic surfactants. The ternary system, surfactant/fatty alcohol/water, leads to a lamellar structure forming a percolated network giving rise to a gel. [2]
See also
- Behentrimonium chloride – an C25 structural analogue
- Cetrimonium bromide – the corresponding bromide salt
References
- ↑ What is cetrimonium chloride? at naturallycurly.com
- ↑ 2.0 2.1 "The cooling process effect on the bilayer phase state of the CTAC/cetearyl alcohol/water surfactant gel", Colloids and Surfaces A 597 (2020) 1248212
 |

