Chemistry:Halazone
| |
| Names | |
|---|---|
| Preferred IUPAC name
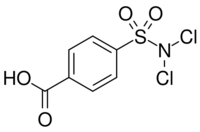
4-(Dichlorosulfamoyl)benzoic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1479 |
| |
| Properties | |
| C7H5Cl2NO4S | |
| Molar mass | 270.08 g·mol−1 |
| Appearance | Fine white powder with an odor of chlorine[2] |
| Melting point | 213 °C (415 °F; 486 K);[3] 196 °C with decomposition.[4] |
| Less than 1 g/L at 70 °F [2] | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319 | |
| P264, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Halazone (4-(dichlorosulfamoyl)benzoic acid) is a chemical compound whose formula can be written as either C7H5Cl2NO4S or (HOOC)(C6H4)(SO2)(NCl2). It has been widely used to disinfect drinking water.
Other names for this compound include p-sulfondichloramidobenzoic acid, 4-[(dichloroamino)sulfonyl]benzoic acid, and Pantocide.
Uses
Halazone tablets have been used to disinfect water for drinking, especially where treated tap water is not available. A typical dosage is 4 mg/L.[5][6]
Halazone tablets were commonly used during World War II by U.S. soldiers for portable water purification, even being included in accessory packs for C-rations until 1945.[7]
Halazone has largely been replaced in that use by sodium dichloroisocyanurate. The primary limitation of halazone tablets was the very short usable life of opened bottles, typically three days or less, unlike iodine-based tablets which have a usable open bottle life of three months.[citation needed]
Dilute halazone solutions (4 to 8 ppm of available chlorine) has also been used to disinfect contact lenses,[8] and as a spermicide.
Mechanism of action
Halazone's disinfecting activity is mainly due to the hypochlorous acid (HClO) released by hydrolysis of the chlorine-nitrogen bonds when the product is dissolved in water:[8]
- (R1)(R2)NCl + H2O → HOCl + (R1)(R2)NH
The hypochlorous acid is a powerful oxidizer and chlorinating agent that destroys or denatures many organic compounds.
Production
Halazone can be prepared by chlorination of p-sulfonamidobenzoic acid.[4]
Another synthesis route is the oxidation of dichloramine-T with potassium permanganate in a mild alkaline medium.[4]
See also
- Bleach
- Chlorine-releasing compound
- Chloramine-T (tosylchloramide sodium salt), another water disinfection agent.
- Water chlorination
References
- ↑ 1.0 1.1 PubChem: "Halazone". Accessed on 2018-06-18.
- ↑ 2.0 2.1 NTP (1992), cited by PubChem
- ↑ Jean-Claude Bradley: Open Melting Point Dataset. Quoted by Chemspider.
- ↑ 4.0 4.1 4.2 Saljoughian, M.; Sadeghi, M. T. (1986). "An improved procedure for the synthesis ofp-(dichlorosulfamoyl)benzoic acid (Halazone)". Monatshefte für Chemie 117 (4): 553. doi:10.1007/BF00810903.
- ↑ Gripo Laboratories: "Water purification range: Halazone USP based Chlorine Tablets ". Product page, accessed on 2018-06-18
- ↑ Precise Health Care PVT LTD: "Halazone tablets ". Product page, accessed on 2018-06-18
- ↑ Hlavatá, L; Aguilaniu, H; Pichová, A; Nyström, T (2003). "The oncogenic RAS2val19 mutation locks respiration, independently of PKA, in a mode prone to generate ROS". The EMBO Journal 22 (13): 3337–3345. doi:10.1093/emboj/cdg314. PMID 12839995.
- ↑ 8.0 8.1 Rosenthal, Ruth Ann; Schlitzen, Ronald L; McNamee, Linda S; Dassanayake, Nissanake L; Amass, Roger (1992). "Antimicrobial activity of organic chlorine releasing compounds". Journal of the British Contact Lens Association 15 (2): 81. doi:10.1016/0141-7037(92)80044-Z.
 |
