Chemistry:Sodium ferrioxalate
| |
| Names | |
|---|---|
| IUPAC name
Sodium iron(III) oxalate, sodium oxalatoferrate, sodium trisoxalatoferrate
| |
| Other names
Sodium ferrioxalate
Sodium ferric oxalate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6FeNa3O12 | |
| Molar mass | 388.868 g·mol−1 |
| Appearance | lime green hydrated crystals |
| Density | 1.97 g/cm3 at 17 °C |
| 32.5pts per 100pts solvent, cold water, 182pts per 100pts, boiling water[1] | |
| Structure | |
| octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | Corrosive. Eye, respiratory and skin irritant. |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312 | |
| P264, P270, P280, P301+312, P302+352, P312, P322, P330, P363, P501 | |
| Related compounds | |
Other anions
|
Potassium ferrioxalate |
Related compounds
|
Iron(II) oxalate Iron(III) oxalate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium ferrioxalate are inorganic compounds with the formula Na
3Fe(C
2O
4)
3(H2O)n. The pentahydrate has been characterized by X-ray crystallography. In contrast the potassium, ammonium, and rubidium salts crystallize from water as their trihydrates.[2]
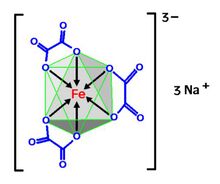
The compound is a salt consisting of ferrioxalate anions, [Fe(C
2O
4)
3]3−, and sodium cations Na+. The anion is a transition metal complex consisting of an iron atom in the +3 oxidation state and three bidentate oxalate ions C
2O2−
4 anions serving as ligands.
The ferrioxalate anion is sensitive to light and higher-energy electromagnetic radiation, which causes the decomposition of one oxalate to carbon dioxide CO
2 and reduction of the iron(III) atom to iron(II).
Preparation

Sodium ferrioxalate can be obtained by mixing solutions of sodium oxalate and ferric oxalate, and waiting a few hours for the brown colour of the ferric oxalate to be replaced with the green colour of the complex anion.
- 3 Na
2C
2O
4 + Fe
2(C
2O
4)
3 → 2 Na
3[Fe(C
2O
4)
3]
The equilibrium is attained only slowly at room temperature. The product can then be crystallized by evaporating the solution at just below boiling until small crystals appear, then allowing it to cool. The product may also be precipitated by adding methanol or ethanol to the solution.
Some decomposition of the ferric oxalate may occur during the process, resulting in the canary-yellow insoluble iron(II) oxalate. Small amounts of hydrogen peroxide H
2O
2 may be added to keep the iron in the 3+ oxidation state.
See also
A number of other iron oxalates are known
- Iron(II) oxalate
- Iron(III) oxalate
- Potassium ferrioxalate
References
- ↑ Weast, Robert C., ed (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-149. ISBN 0-8493-0462-8.
- ↑ Armentano, Donatella; De Munno, Giovanni; Lloret, Francesc; Julve, Miguel (2005). "Bis and tris(oxalato)ferrate(III) complexes as precursors of polynuclear compounds". CrystEngComm 7 (7): 57. doi:10.1039/b417251e.
 |
