Chemistry:Hydrogen ozonide
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
Hydrogen ozonide
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| HO3 | |
| Molar mass | 49.005 g·mol−1 |
| Related compounds | |
Related hydrogen polyoxides
|
|
Related compounds
|
Protonated ozone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
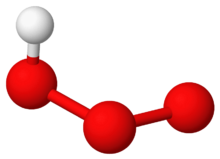
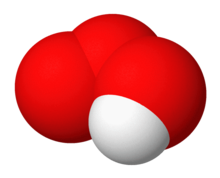
Hydrogen ozonide (HO
3) is a radical molecule consisting of a hydrogen atom covalently bonded to an ozonide unit.[1]
It is possibly produced in the reaction of the hydroxyl radical with dioxygen: OH• + O2 → HO3•.[2][3]
It has been detected in a mass spectrometer experiment using HO+3 (protonated ozone) as precursor.[4]
References
- ↑ Möller, D. (2022). Chemistry for Environmental Scientists. De Gruyter Textbook. De Gruyter. p. 165. ISBN 978-3-11-073517-8. https://books.google.com/books?id=BfqjEAAAQBAJ&pg=PA165. Retrieved 2022-12-28.
- ↑ Wabner, Dietrich; Grambow, Clemens (November 1985). "Reactive intermediates during oxindation of water lead dioxide and platinum electrodes". Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 195 (1): 95–108. doi:10.1016/0022-0728(85)80008-5.
- ↑ Chernik, A.; Drozdovich, V. B.; Zharskii, I. (1997). "Ozone evolution at the lead dioxide electrode in highly acid and neutral electrolytes : The influence of polarization and fluoride ions on the process kinetics" (in en). Russian Journal of Electrochemistry 33 (3): 259–263.
- ↑ Cacace, Fulvio; de Petris, Guilia; Pepi, F.; Troiani, Anna (2 July 1999). "Experimental Detection of Hydrogen Trioxide". Science 285 (5424): 81–82. doi:10.1126/science.285.5424.81.
Extra reading
- Kalemos, Apostolos (1 February 2021). "Some ab initio thoughts on the bonding in O3H". Molecular Physics 119 (3): e1804082. doi:10.1080/00268976.2020.1804082.
- Fabian, W. M. F.; Kalcher, J.; Janoschek, R. (September 2005). "Stationary points on the energy hypersurface of the reaction O3 + H•→ [•O3H]* ⇆ O2 + •OH and thermodynamic functions of •O3H at G3MP2B3, CCSD(T)–CBS (W1U) and MR–ACPF–CBS levels of theory". Theoretical Chemistry Accounts 114 (1–3): 182–188. doi:10.1007/s00214-005-0659-7.
